What is SN2? SN2 stands for bimolecular nucleophilic substitution. It's a type of chemical reaction where a nucleophile attacks an electrophile, leading to the replacement of a leaving group. This reaction is crucial in organic chemistry because it helps form new bonds and create different molecules. Why is it important? SN2 reactions are essential for synthesizing various compounds, including pharmaceuticals, agrochemicals, and polymers. How does it work? The nucleophile attacks the electrophile from the opposite side of the leaving group, resulting in a single-step mechanism. What makes it unique? The reaction rate depends on both the nucleophile and the electrophile, making it a second-order reaction. Understanding SN2 reactions can help you grasp more complex chemical processes and their applications.
What is SN2?
SN2 stands for bimolecular nucleophilic substitution. It's a type of chemical reaction where a nucleophile replaces a leaving group in a molecule. This reaction is crucial in organic chemistry, especially for synthesizing various compounds.
-
SN2 reactions are second-order reactions. The rate depends on the concentration of both the nucleophile and the substrate.
-
The reaction mechanism involves a single, concerted step. Both bond formation and bond breaking occur simultaneously.
-
SN2 stands for Substitution Nucleophilic Bimolecular. This name reflects the two molecules involved in the rate-determining step.
-
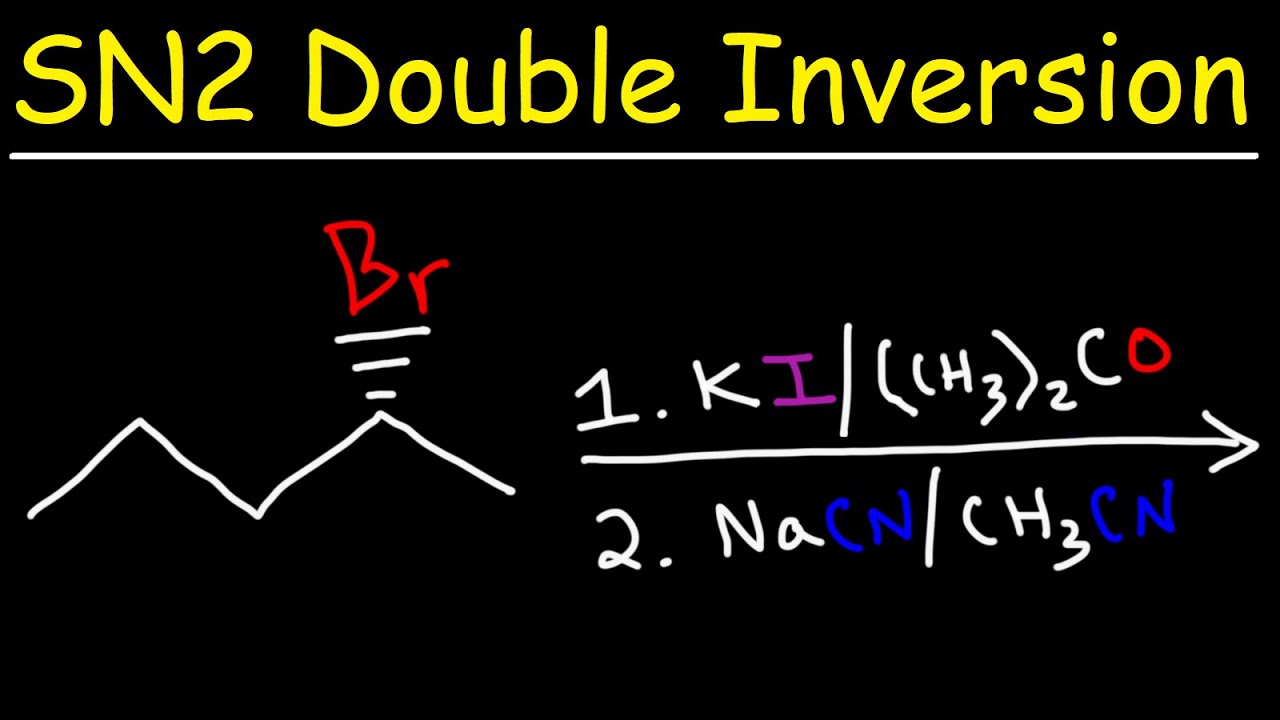
The nucleophile attacks from the opposite side of the leaving group. This backside attack leads to an inversion of configuration at the carbon center.
-
Steric hindrance affects SN2 reactions. Bulky groups around the carbon center slow down or prevent the reaction.
Key Players in SN2 Reactions
Understanding the components involved in SN2 reactions helps grasp their dynamics better. The nucleophile, substrate, and leaving group all play vital roles.
-
A strong nucleophile is essential. Good nucleophiles are typically negatively charged or neutral molecules with lone pairs of electrons.
-
Primary alkyl halides react faster than secondary or tertiary ones. Less steric hindrance around the carbon center makes the reaction more efficient.
-
The leaving group must be stable once it departs. Good leaving groups can stabilize the negative charge after leaving the substrate.
-
Polar aprotic solvents are ideal for SN2 reactions. These solvents do not form hydrogen bonds with the nucleophile, allowing it to stay reactive.
-
Common nucleophiles include OH-, CN-, and NH3. These molecules are highly reactive and can easily attack the carbon center.
Reaction Conditions and Influences
Several factors influence the rate and outcome of SN2 reactions. These include temperature, solvent, and the nature of the nucleophile and substrate.
-
Higher temperatures generally increase reaction rates. More kinetic energy helps overcome activation energy barriers.
-
Solvent choice can make or break the reaction. Polar aprotic solvents like DMSO and acetone are preferred.
-
Steric effects are a major consideration. Bulky groups around the carbon center hinder the nucleophile's approach.
-
The nature of the leaving group is crucial. Better leaving groups like iodide or bromide facilitate faster reactions.
-
The nucleophile's strength and concentration matter. Stronger and more concentrated nucleophiles increase the reaction rate.
Real-World Applications
SN2 reactions are not just theoretical; they have practical applications in various fields, from pharmaceuticals to materials science.
-
SN2 reactions are used in drug synthesis. Many pharmaceuticals are created through these reactions.
-
They are essential in the production of agrochemicals. Pesticides and herbicides often involve SN2 mechanisms.
-
SN2 reactions help in creating polymers. Some polymerization processes rely on these reactions.
-
They are used in the synthesis of fragrances and flavors. Many aromatic compounds are produced through SN2 mechanisms.
-
SN2 reactions are crucial in biochemistry. Enzyme-catalyzed reactions often follow SN2 pathways.
Challenges and Limitations
While SN2 reactions are versatile, they come with their own set of challenges and limitations.
-
Steric hindrance can be a significant barrier. Bulky substrates react very slowly or not at all.
-
Competing reactions can occur. SN1 reactions might compete under certain conditions, leading to different products.
-
The reaction is sensitive to the solvent used. Using the wrong solvent can drastically reduce the reaction rate.
-
Temperature control is crucial. Too high or too low temperatures can affect the reaction outcome.
-
The reaction is not suitable for all substrates. Only certain types of molecules can undergo SN2 reactions efficiently.
Historical Context
The understanding of SN2 reactions has evolved over time, thanks to contributions from various scientists.
-
The concept was first introduced by Edward Hughes and Christopher Ingold. They proposed the mechanism in the 1930s.
-
The term SN2 was coined in the mid-20th century. It helped standardize the nomenclature in organic chemistry.
-
Early studies focused on simple alkyl halides. These provided a clear understanding of the reaction mechanism.
-
Advancements in spectroscopy helped elucidate the reaction pathway. Techniques like NMR and IR spectroscopy provided insights.
-
Modern computational chemistry has refined our understanding. Simulations and models offer detailed views of the reaction dynamics.
Fun Facts About SN2
Beyond the technical details, SN2 reactions have some interesting and lesser-known aspects.
-
SN2 reactions can be used to create chiral centers. This is important in producing enantiomerically pure compounds.
-
The reaction is stereospecific. It always leads to an inversion of configuration at the carbon center.
-
SN2 reactions are faster in gas phase than in solution. The absence of solvent molecules reduces steric hindrance.
-
They can occur in biological systems. Some enzyme-catalyzed reactions follow SN2 mechanisms.
-
SN2 reactions are used in forensic science. They help in the synthesis of certain chemical markers.
-
The reaction can be visualized using molecular models. This helps in teaching and understanding the mechanism.
-
SN2 reactions are a staple in organic chemistry textbooks. They are one of the first mechanisms students learn.
-
The reaction has inspired numerous research studies. Scientists continue to explore its nuances and applications.
The Final Word on SN2 Reactions
SN2 reactions are fascinating. They involve a backside attack, leading to an inversion of configuration. These reactions are bimolecular, meaning both the nucleophile and substrate play crucial roles. Factors like the strength of the nucleophile, the nature of the leaving group, and the solvent can significantly impact the reaction rate. Polar aprotic solvents are ideal for SN2 reactions because they don't solvate the nucleophile, making it more reactive. Primary alkyl halides are more reactive in SN2 reactions compared to secondary and tertiary ones due to less steric hindrance. Understanding these key points helps in predicting and controlling the outcomes of SN2 reactions in various chemical processes. Whether you're a student, a researcher, or just curious about chemistry, knowing these facts can deepen your appreciation for the intricate dance of atoms and molecules.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
