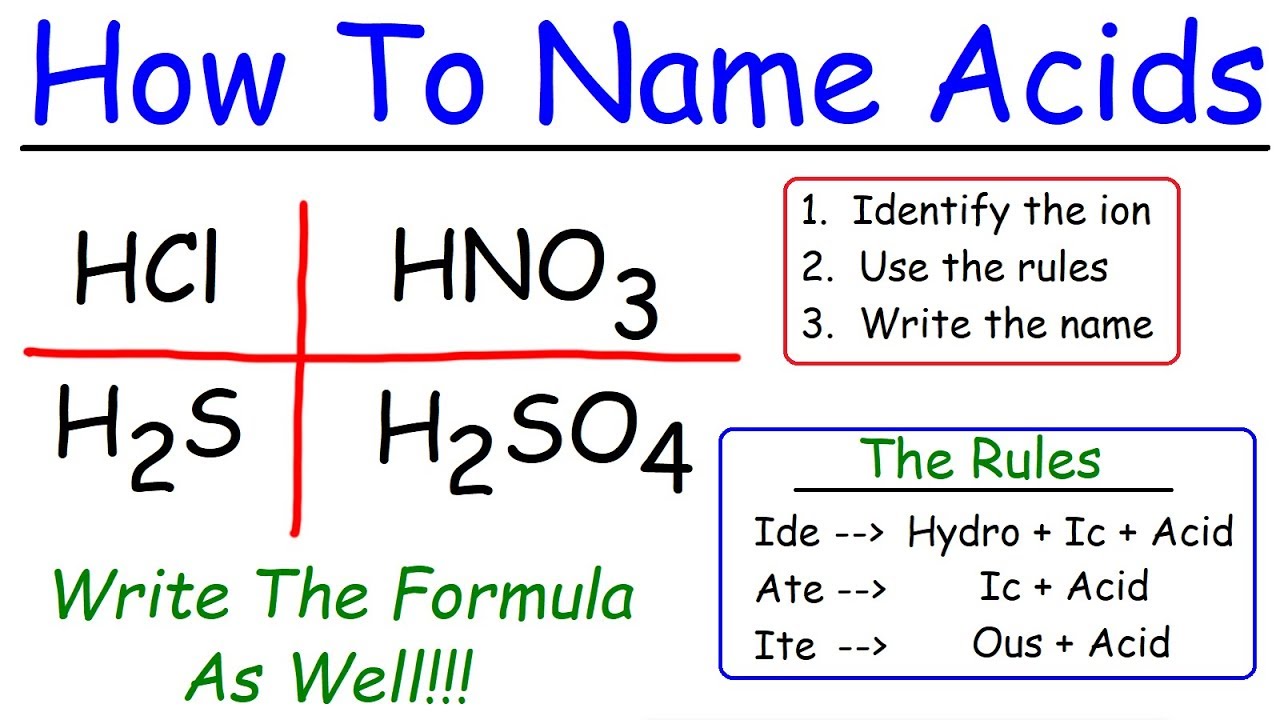
Naming acids can seem tricky, but it’s simpler than you might think. Acids are substances that release hydrogen ions (H⁺) when dissolved in water. The name of an acid depends on its anion, the negatively charged part. For instance, if the anion ends in "-ide," the acid name starts with "hydro-" and ends with "-ic acid." For anions ending in "-ate," the acid name ends in "-ic acid," while those ending in "-ite" change to "-ous acid." Understanding these rules helps in identifying and naming acids correctly. Ready to dive into the world of acids? Let’s get started!
Understanding Acids
Acids are fascinating substances that play a crucial role in chemistry and everyday life. Let's dive into some intriguing facts about naming acids.
-
Acids are named based on their anions. The name of an acid is derived from the name of the anion it forms when dissolved in water.
-
Binary acids contain hydrogen and one other element. These acids are named with the prefix "hydro-" followed by the root of the nonmetal element and the suffix "-ic."
-
Oxyacids contain hydrogen, oxygen, and another element. The name of an oxyacid is based on the polyatomic ion it contains. If the ion ends in "-ate," the acid name ends in "-ic." If the ion ends in "-ite," the acid name ends in "-ous."
-
Hydrochloric acid is a binary acid. Formed from hydrogen and chlorine, its chemical formula is HCl.
-
Sulfuric acid is an oxyacid. Derived from the sulfate ion (SO₄²⁻), its chemical formula is H₂SO₄.
Common Acids and Their Names
Many acids are commonly encountered in both laboratory and household settings. Here are some well-known examples.
-
Nitric acid (HNO₃) is a strong acid. It is derived from the nitrate ion (NO₃⁻).
-
Acetic acid (CH₃COOH) is found in vinegar. It is an organic acid with the acetate ion (CH₃COO⁻).
-
Phosphoric acid (H₃PO₄) is used in soft drinks. It comes from the phosphate ion (PO₄³⁻).
-
Carbonic acid (H₂CO₃) forms in carbonated beverages. It is derived from the carbonate ion (CO₃²⁻).
-
Hydrofluoric acid (HF) is used to etch glass. It is a binary acid formed from hydrogen and fluorine.
Naming Rules and Exceptions
While naming acids follows specific rules, there are always exceptions and special cases.
-
Some acids have common names. For example, acetic acid is commonly known as vinegar.
-
Old names are sometimes still used. For instance, muriatic acid is an old name for hydrochloric acid.
-
Polyprotic acids can donate more than one proton. Examples include sulfuric acid (H₂SO₄) and phosphoric acid (H₃PO₄).
-
Formic acid (HCOOH) is named after ants. The name comes from the Latin word "formica," meaning ant, as it was first isolated from ant bodies.
-
Citric acid is found in citrus fruits. Its chemical formula is C₆H₈O₇.
Historical and Fun Facts
Acids have a rich history and some interesting trivia associated with them.
-
The word "acid" comes from the Latin "acidus." It means sour, reflecting the taste of many acids.
-
Alchemists were the first to study acids. They discovered many acids through their experiments.
-
Sulfuric acid was once called "oil of vitriol." This name was used by alchemists.
-
Lavoisier named oxygen, thinking it was essential for all acids. He was partially correct, as many acids do contain oxygen.
-
Hydrochloric acid was discovered by Jabir ibn Hayyan. This Persian alchemist is often called the father of chemistry.
Industrial and Everyday Uses
Acids are not just for the lab; they have numerous applications in industry and daily life.
-
Sulfuric acid is used in car batteries. It acts as the electrolyte in lead-acid batteries.
-
Nitric acid is used in fertilizers. It is a key component in the production of ammonium nitrate.
-
Acetic acid is used in food preservation. It is a common ingredient in pickling.
-
Citric acid is a natural preservative. It is also used to add a sour taste to foods and beverages.
-
Hydrochloric acid is used in cleaning. It is effective in removing rust and scale from metals.
Safety and Handling
Acids can be dangerous if not handled properly. Here are some important safety tips.
-
Always wear protective gear. This includes gloves, goggles, and lab coats.
-
Work in a well-ventilated area. Many acids release harmful fumes.
-
Never add water to acid. Always add acid to water to prevent splashing.
-
Store acids in appropriate containers. Use containers made of materials that resist corrosion.
-
Know the emergency procedures. Be prepared to neutralize spills and provide first aid.
Environmental Impact
Acids can have significant effects on the environment.
-
Acid rain is caused by sulfuric and nitric acids. These acids form when sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) react with water in the atmosphere.
-
Acid rain harms aquatic life. It lowers the pH of water bodies, affecting fish and other organisms.
-
Soil acidification affects plant growth. Acidic soils can limit nutrient availability to plants.
-
Industrial emissions contribute to acid rain. Reducing these emissions can help mitigate the problem.
-
Limestone can neutralize acid rain. It is often used to treat affected lakes and soils.
Interesting Chemical Properties
Acids exhibit unique chemical behaviors that make them useful in various applications.
-
Acids react with bases to form salts. This reaction is called neutralization.
-
Acids can conduct electricity. They ionize in water, allowing the solution to carry an electric current.
Final Thoughts on Naming Acids
Naming acids might seem tricky at first, but with a bit of practice, it becomes second nature. Remember, binary acids start with "hydro-" and end with "-ic," while oxyacids depend on the number of oxygen atoms. If there's one less oxygen, the suffix changes to "-ous." For those with even fewer oxygens, use the prefix "hypo-" and the suffix "-ous." On the flip side, if there's one more oxygen than the common form, add the prefix "per-" and keep the "-ic" ending.
Understanding these patterns helps in mastering the naming conventions. Whether you're a student, a teacher, or just curious, these rules simplify the process. Keep practicing, and soon enough, you'll name acids without a second thought. Happy learning!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
