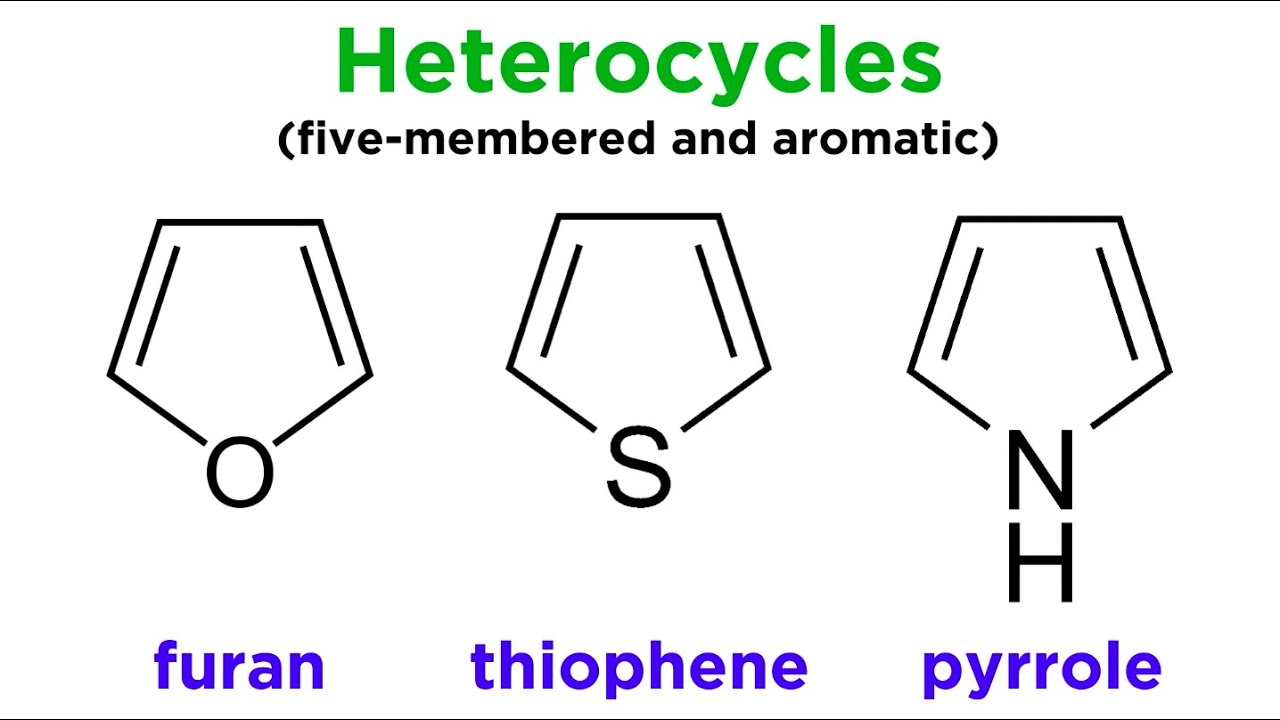
What is thiophene? Thiophene is a sulfur-containing heterocyclic compound with a five-membered ring structure. Why is thiophene important? This organic compound plays a crucial role in various fields, including pharmaceuticals, materials science, and organic electronics. Where is thiophene found? It naturally occurs in coal tar and petroleum. How is thiophene used? It's used to create conductive polymers, which are essential in making electronic devices like OLEDs and solar cells. What makes thiophene unique? Its aromatic stability and ability to conduct electricity set it apart from other compounds. Can thiophene be synthesized? Yes, it can be synthesized through several chemical reactions, making it versatile for industrial applications. Is thiophene safe? While generally stable, it should be handled with care due to potential health risks.
What is Thiophene?
Thiophene is a fascinating organic compound with a five-membered ring structure. It contains four carbon atoms and one sulfur atom. This compound is part of a larger family known as heterocyclic compounds. Let's dive into some intriguing facts about thiophene.
-
Thiophene's molecular formula is C4H4S. This simple formula hides a world of complexity and utility in various scientific fields.
-
Discovered in 1883 by Viktor Meyer. Meyer found thiophene while studying benzene. He noticed impurities in benzene derived from coal tar, leading to the discovery of thiophene.
-
Thiophene smells like benzene. Despite containing sulfur, thiophene has a sweet, benzene-like odor, making it less offensive than many sulfur compounds.
-
Used in the production of pharmaceuticals. Thiophene derivatives are crucial in creating drugs, including antibiotics and anti-inflammatory medications.
-
Found in crude oil. Thiophene naturally occurs in crude oil, contributing to the sulfur content that must be removed during refining.
Chemical Properties of Thiophene
Understanding thiophene's chemical properties helps in grasping its applications and behavior in different environments.
-
Thiophene is aromatic. Like benzene, thiophene exhibits aromaticity, providing stability and unique chemical reactivity.
-
Resistant to hydrogenation. Thiophene's aromatic nature makes it less reactive to hydrogenation compared to non-aromatic compounds.
-
Undergoes electrophilic substitution reactions. This property allows thiophene to participate in various chemical reactions, making it versatile in organic synthesis.
-
Thiophene is slightly soluble in water. Its solubility in water is limited, but it dissolves well in organic solvents like ethanol and ether.
-
Boiling point is 84°C. This relatively low boiling point makes thiophene easy to handle in laboratory settings.
Applications of Thiophene
Thiophene's unique properties make it valuable in multiple industries, from pharmaceuticals to electronics.
-
Used in organic semiconductors. Thiophene derivatives are essential in developing organic semiconductors for electronic devices.
-
Component in conducting polymers. Polythiophene, a polymer of thiophene, is used in creating conductive materials for various applications.
-
Involved in dye production. Thiophene derivatives contribute to the synthesis of dyes and pigments used in textiles and printing.
-
Used in agrochemicals. Thiophene compounds are found in pesticides and herbicides, aiding in agricultural productivity.
-
Essential in flavor and fragrance industry. Some thiophene derivatives are used to create flavors and fragrances, adding unique scents and tastes.
Environmental Impact of Thiophene
While thiophene has many uses, its presence in the environment can have significant effects.
-
Contributes to sulfur emissions. When fuels containing thiophene are burned, sulfur dioxide is released, contributing to air pollution.
-
Biodegradable under certain conditions. Some bacteria can break down thiophene, reducing its environmental impact.
-
Present in cigarette smoke. Thiophene is one of the many compounds found in cigarette smoke, contributing to its harmful effects.
-
Monitored in water sources. Due to its potential toxicity, thiophene levels are monitored in water sources to ensure safety.
-
Used in environmental research. Thiophene serves as a model compound in studying the behavior of sulfur-containing pollutants.
Interesting Facts About Thiophene
Beyond its practical applications, thiophene has some quirky and lesser-known aspects.
-
Thiophene can form complexes with metals. These complexes are studied for their potential catalytic properties.
-
Used in the study of aromaticity. Thiophene is often used as a model compound in research on aromaticity and related phenomena.
-
Thiophene derivatives can be fluorescent. Some thiophene compounds exhibit fluorescence, useful in scientific imaging techniques.
-
Found in outer space. Thiophene has been detected in the interstellar medium, indicating its presence beyond Earth.
-
Used in organic solar cells. Thiophene-based materials are explored for their potential in improving solar cell efficiency.
Health and Safety Aspects of Thiophene
Handling thiophene requires understanding its health and safety implications.
-
Thiophene is flammable. It can ignite easily, necessitating careful handling and storage.
-
Can cause skin irritation. Direct contact with thiophene may lead to skin irritation or allergic reactions.
-
Inhalation risks. Breathing in thiophene vapors can cause respiratory irritation and other health issues.
-
Regulated by safety guidelines. Various safety guidelines exist to ensure the safe handling and disposal of thiophene.
-
Used in research on toxicity. Thiophene is studied to understand its toxicological effects and develop safety protocols.
Thiophene's versatility and unique properties make it a compound of great interest across multiple fields. From its discovery in the 19th century to its modern applications, thiophene continues to be a subject of scientific fascination.
The Final Word on Thiophene
Thiophene, a sulfur-containing compound, plays a crucial role in various fields. From its discovery in coal tar to its applications in pharmaceuticals, electronics, and materials science, thiophene's versatility is impressive. Its unique properties make it a valuable component in organic semiconductors, conductive polymers, and even cancer treatment research.
Understanding thiophene's structure and reactivity helps scientists develop new materials and technologies. Its presence in natural products and fossil fuels highlights its importance in both biological and industrial processes.
As research continues, thiophene's potential applications will likely expand, offering new solutions to complex problems. Whether you're a chemistry enthusiast or just curious about the world around you, knowing these facts about thiophene can deepen your appreciation for this fascinating compound. Keep an eye on future developments, as thiophene's story is far from over.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
