What is molar mass? Molar mass is the mass of one mole of a substance, usually measured in grams per mole (g/mol). It's a crucial concept in chemistry, helping scientists and students alike to understand the amount of substance present in a given sample. By knowing the molar mass, you can easily convert between the mass of a substance and the number of moles, making it simpler to perform chemical calculations and reactions. For example, the molar mass of water (H₂O) is approximately 18 g/mol, meaning one mole of water weighs 18 grams. Understanding molar mass can make chemistry more approachable and less intimidating.
What is Molar Mass?
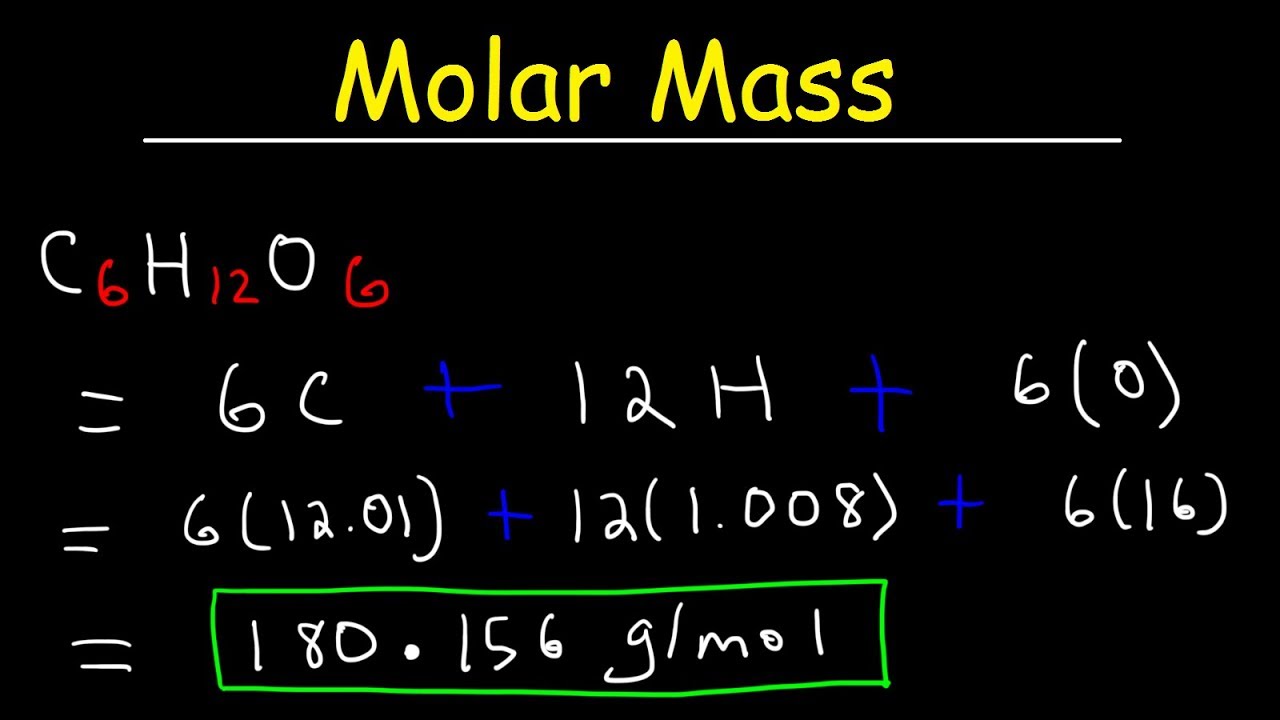
Molar mass is a fundamental concept in chemistry. It represents the mass of one mole of a given substance, typically measured in grams per mole (g/mol). Understanding molar mass helps in various chemical calculations and reactions.
- Molar mass is the mass of one mole of a substance.
- It is measured in grams per mole (g/mol).
- Molar mass is calculated by summing the atomic masses of all atoms in a molecule.
- The atomic mass of an element is found on the periodic table.
- For example, the molar mass of water (H₂O) is approximately 18.015 g/mol.
Importance of Molar Mass in Chemistry
Molar mass plays a crucial role in stoichiometry, which involves calculating the quantities of reactants and products in chemical reactions. It helps chemists understand how much of each substance is needed or produced.
- Molar mass is essential for stoichiometric calculations.
- It helps determine the proportions of reactants and products in a reaction.
- Molar mass is used to convert between grams and moles.
- It aids in balancing chemical equations.
- Chemists use molar mass to calculate the yield of a reaction.
How to Calculate Molar Mass
Calculating molar mass involves adding up the atomic masses of all the atoms in a molecule. This process is straightforward but requires attention to detail.
- Identify the chemical formula of the substance.
- Find the atomic masses of each element in the formula.
- Multiply the atomic mass of each element by the number of atoms of that element in the molecule.
- Sum the total masses to get the molar mass.
- For example, the molar mass of CO₂ is calculated as follows: C (12.01 g/mol) + 2 × O (16.00 g/mol) = 44.01 g/mol.
Applications of Molar Mass
Molar mass is not just a theoretical concept; it has practical applications in various fields, including pharmaceuticals, environmental science, and materials science.
- In pharmaceuticals, molar mass helps determine drug dosages.
- Environmental scientists use molar mass to measure pollutant concentrations.
- Molar mass is crucial in materials science for developing new materials.
- It aids in the calculation of molar concentrations in solutions.
- Molar mass is used in gas laws to relate volume, pressure, and temperature.
Interesting Facts About Molar Mass
Molar mass might seem like a dry topic, but it has some fascinating aspects that can spark curiosity.
- The concept of the mole was introduced by Amedeo Avogadro.
- One mole contains exactly 6.02214076 × 10²³ particles, known as Avogadro's number.
- The molar mass of a substance is numerically equal to its molecular or formula mass in atomic mass units (amu).
- Heavy water (D₂O) has a higher molar mass than regular water (H₂O) due to the presence of deuterium.
- The molar mass of a compound can affect its physical properties, such as boiling and melting points.
Common Mistakes in Calculating Molar Mass
Even though calculating molar mass is straightforward, common mistakes can lead to incorrect results. Being aware of these pitfalls can improve accuracy.
- Forgetting to multiply the atomic mass by the number of atoms in the molecule.
- Using incorrect atomic masses from outdated periodic tables.
- Misidentifying the chemical formula of the substance.
- Ignoring significant figures in calculations.
- Confusing molar mass with molecular weight, which are related but not identical concepts.
Final Thoughts on Molar Mass
Understanding molar mass is crucial for anyone diving into chemistry. It helps in calculating the amount of substances involved in reactions, making it a fundamental concept. Knowing how to find the molar mass of elements and compounds can simplify complex problems, whether you're a student or a professional.
Remember, the molar mass is the mass of one mole of a substance, typically measured in grams per mole. It’s derived from the atomic masses of the elements in the periodic table. This knowledge can aid in various scientific calculations, from determining the yield of a reaction to figuring out the concentration of a solution.
Grasping this concept can make chemistry less intimidating and more accessible. Keep practicing, and soon, calculating molar mass will become second nature. Happy studying!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
