Cyanogen azide is a chemical compound that might sound like something out of a sci-fi movie, but it’s very real and quite interesting. Known for its explosive properties, this compound has a formula of C2N4. It’s a colorless, volatile liquid that can be quite dangerous if not handled properly. But what makes cyanogen azide so fascinating? Why is it important in the world of chemistry? In this blog post, we’ll dive into 30 intriguing facts about cyanogen azide, from its discovery to its various uses and the precautions needed when dealing with it. Get ready to learn about one of chemistry’s most captivating compounds!
Key Takeaways:
- Cyanogen azide is a highly reactive compound with potential applications in explosives, pharmaceuticals, and material science. However, its hazardous nature requires careful handling and strict safety measures.
- Despite its fascinating properties, cyanogen azide poses environmental risks and strict regulations are in place to protect the environment from its toxic and reactive nature.
What is Cyanogen Azide?
Cyanogen azide is a fascinating chemical compound with a unique structure and properties. It has intrigued scientists for years due to its reactivity and potential applications. Here are some interesting facts about this compound.
-
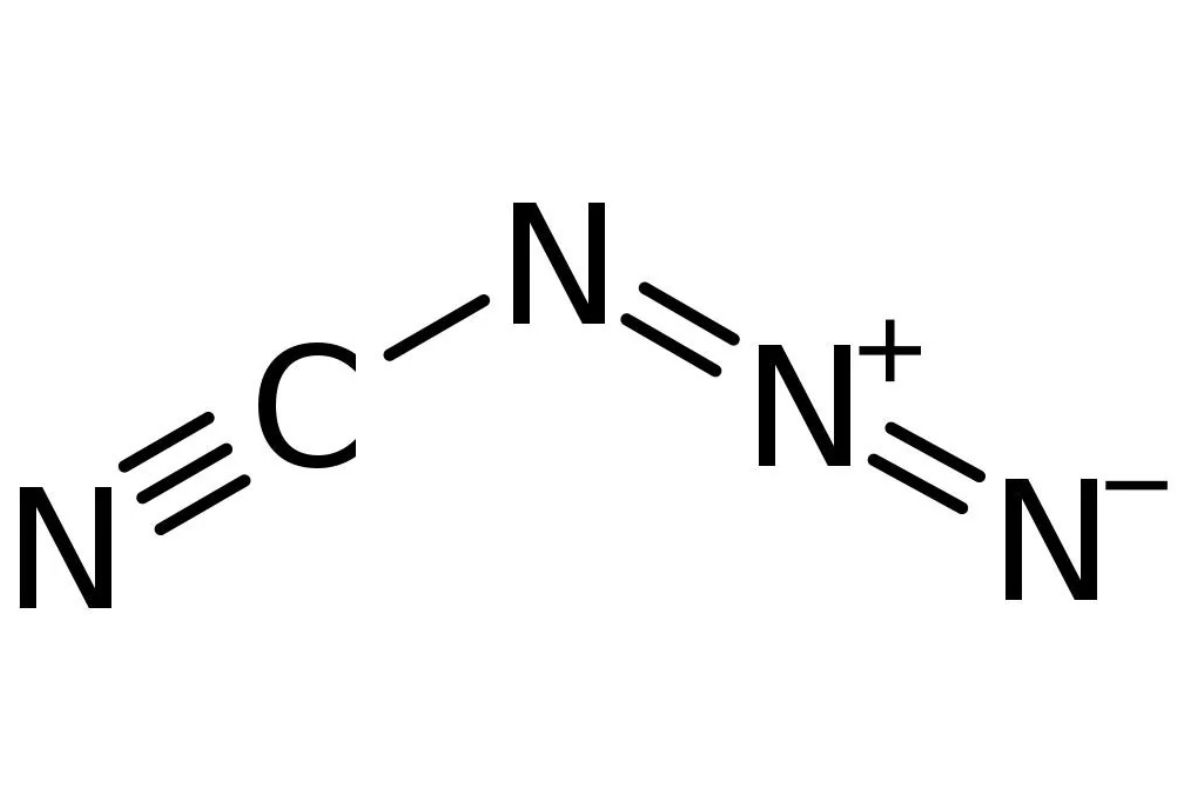
Chemical Formula: The chemical formula for cyanogen azide is NCN3. This simple yet powerful formula hints at its reactivity.
-
Molecular Structure: It consists of a cyanogen group (NC) bonded to an azide group (N3). This combination makes it highly reactive.
-
Discovery: Cyanogen azide was first synthesized in the early 20th century. Its discovery opened new avenues in the study of azides.
-
Appearance: It is a colorless, volatile liquid at room temperature. Its appearance can be deceiving given its potent properties.
-
Odor: Cyanogen azide has a pungent smell. This strong odor is a warning of its potential hazards.
Chemical Properties of Cyanogen Azide
Understanding the chemical properties of cyanogen azide helps in grasping its behavior in various reactions. Here are some key properties.
-
Reactivity: It is highly reactive and can decompose explosively. This reactivity is due to the presence of the azide group.
-
Stability: Cyanogen azide is unstable and can decompose under certain conditions. This instability requires careful handling.
-
Solubility: It is soluble in organic solvents like acetone and ether. This solubility makes it useful in various chemical reactions.
-
Boiling Point: The boiling point of cyanogen azide is around 52°C (126°F). This low boiling point contributes to its volatility.
-
Melting Point: It has a melting point of -80°C (-112°F). This low melting point indicates its liquid state at room temperature.
Uses and Applications
Despite its hazardous nature, cyanogen azide has several applications in scientific research and industry. Here are some notable uses.
-
Explosives: It is used in the synthesis of explosives. Its high reactivity makes it suitable for this purpose.
-
Chemical Synthesis: Cyanogen azide is a valuable reagent in organic synthesis. It helps in the formation of various nitrogen-containing compounds.
-
Pharmaceuticals: It is used in the development of certain pharmaceuticals. Its ability to introduce azide groups is beneficial in drug synthesis.
-
Material Science: Researchers use it in the study of new materials. Its unique properties aid in the development of advanced materials.
-
Biochemistry: Cyanogen azide is used in biochemical research. It helps in the study of enzyme mechanisms and protein structures.
Safety and Handling
Due to its hazardous nature, proper safety measures are crucial when handling cyanogen azide. Here are some important safety facts.
-
Toxicity: Cyanogen azide is highly toxic. Inhalation or ingestion can be fatal.
-
Protective Gear: Handling requires the use of protective gear, including gloves and goggles. This gear helps prevent exposure.
-
Storage: It should be stored in a cool, dry place away from light. Proper storage minimizes the risk of decomposition.
-
Ventilation: Work with cyanogen azide should be done in a well-ventilated area. Good ventilation reduces the risk of inhalation.
-
Emergency Procedures: Knowledge of emergency procedures is essential. Quick action can mitigate the effects of accidental exposure.
Environmental Impact
The environmental impact of cyanogen azide is a concern due to its toxicity and reactivity. Here are some environmental facts.
-
Decomposition Products: Its decomposition can release toxic gases. These gases can harm the environment.
-
Water Contamination: Cyanogen azide can contaminate water sources. This contamination poses a risk to aquatic life.
-
Air Pollution: It can contribute to air pollution if not handled properly. Proper disposal is necessary to prevent this.
-
Soil Contamination: Spills can lead to soil contamination. This contamination can affect plant and animal life.
-
Regulations: There are strict regulations regarding its use and disposal. These regulations help protect the environment.
Interesting Facts
Beyond its chemical properties and applications, cyanogen azide has some intriguing aspects. Here are a few interesting facts.
-
Historical Use: During World War II, it was studied for potential use in chemical warfare. Its high toxicity made it a candidate.
-
Research Tool: Scientists use it as a tool to study reaction mechanisms. Its reactivity provides insights into chemical processes.
-
Synthesis Challenges: Synthesizing cyanogen azide requires careful control of conditions. Small deviations can lead to dangerous outcomes.
-
Spectroscopy: It has unique spectral properties. These properties make it useful in spectroscopic studies.
-
Future Potential: Ongoing research explores new applications for cyanogen azide. Its unique properties continue to intrigue scientists.
Final Thoughts on Cyanogen Azide
Cyanogen azide is a fascinating compound with a rich history and unique properties. Its explosive nature makes it both dangerous and intriguing. Scientists have studied it extensively to understand its behavior and potential applications. Despite its risks, cyanogen azide has found uses in various fields, including organic synthesis and materials science. Handling this compound requires extreme caution due to its instability and sensitivity to shock. Proper safety measures are crucial when working with cyanogen azide to prevent accidents. Its role in advancing scientific knowledge cannot be understated, as it continues to be a subject of research and interest. Understanding cyanogen azide helps us appreciate the complexities of chemistry and the importance of safety in scientific endeavors. Whether you're a chemistry enthusiast or just curious, learning about cyanogen azide offers a glimpse into the world of high-energy compounds and their impact on science.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
