Actinium(III) chloride might sound like something out of a sci-fi movie, but it's a real chemical compound with some pretty cool facts. This compound, with the formula AcCl3, is a white, crystalline solid that plays a role in various scientific fields. Ever wondered what makes it special? For starters, it's radioactive, which means it can glow in the dark under certain conditions. Scientists often use it in research related to nuclear energy and radiation. Curious about its history? Actinium was discovered in 1899, and its chloride form has been studied ever since. Ready to learn more? Let's dive into 30 fascinating facts about this intriguing compound!
Key Takeaways:
- Actinium(III) Chloride: A Radioactive Marvel Actinium(III) chloride is a rare, radioactive compound with unique properties. It's used in cancer treatment and scientific research, but handling it requires strict safety measures due to its radioactivity.
- Environmental Impact of Actinium(III) Chloride Improper disposal of actinium(III) chloride can lead to radioactive contamination. Strict regulations and containment measures are in place to protect the environment from its potential impact.
What is Actinium(III) Chloride?
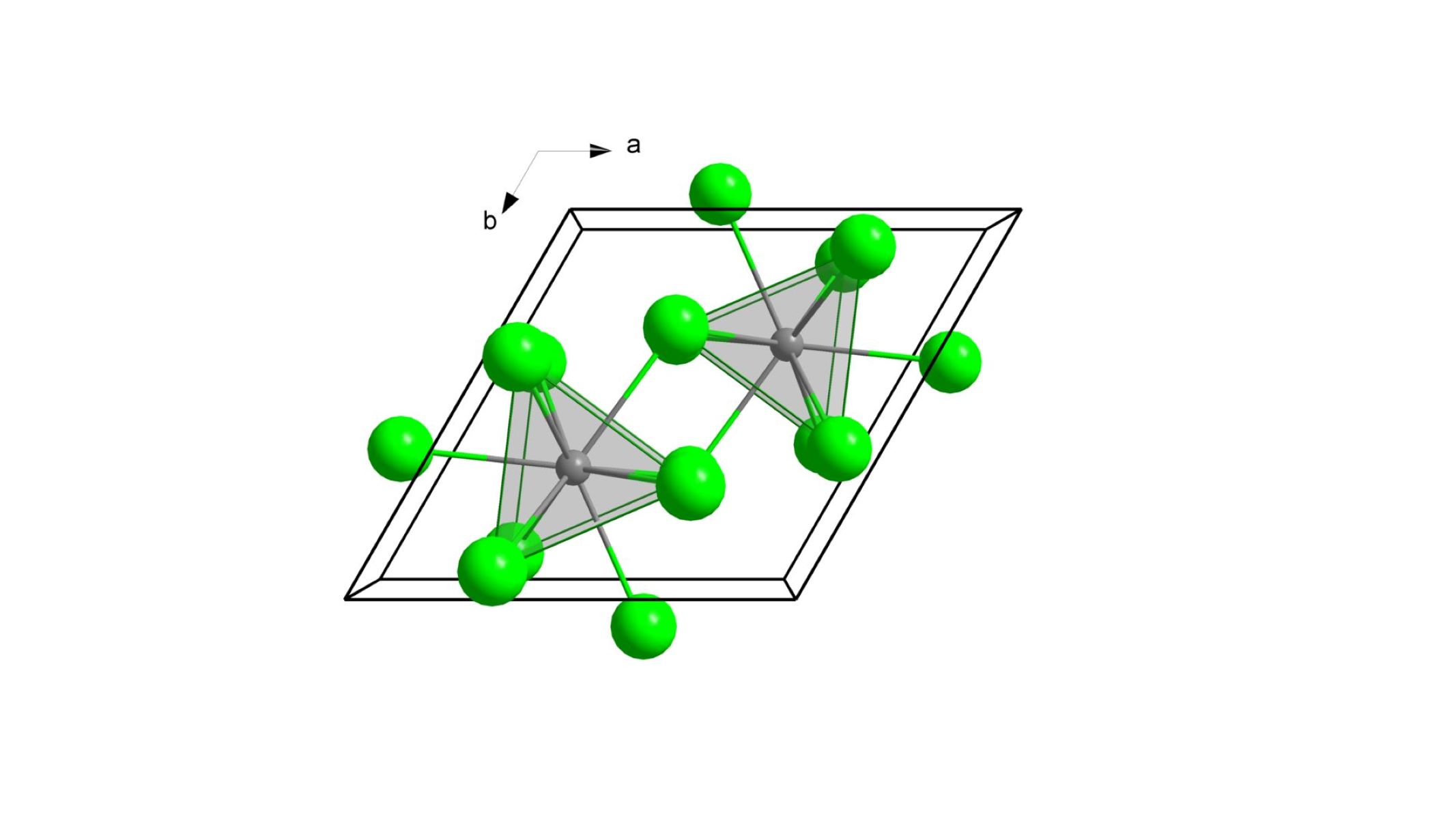
Actinium(III) chloride is a chemical compound with the formula AcCl₃. It consists of actinium and chlorine atoms. This compound is known for its unique properties and uses in various scientific fields. Let's dive into some fascinating facts about this intriguing substance.
Basic Properties of Actinium(III) Chloride
Understanding the basic properties of actinium(III) chloride helps in grasping its significance.
- Chemical Formula: The chemical formula for actinium(III) chloride is AcCl₃.
- Appearance: It appears as a white crystalline solid.
- Molar Mass: The molar mass of actinium(III) chloride is approximately 339.48 g/mol.
- Melting Point: This compound has a melting point of around 850°C.
- Solubility: Actinium(III) chloride is soluble in water, forming a clear solution.
Historical Context
Actinium(III) chloride has an interesting history tied to the discovery of actinium itself.
- Discovery of Actinium: Actinium was discovered in 1899 by Friedrich Oskar Giesel.
- First Synthesis: The first synthesis of actinium(III) chloride was achieved in the early 20th century.
- Early Research: Initial research focused on understanding the radioactive properties of actinium compounds.
Radioactive Nature
Actinium(III) chloride is known for its radioactive properties, which have various applications.
- Radioactivity: Actinium is a highly radioactive element, and actinium(III) chloride inherits this property.
- Alpha Emission: It primarily emits alpha particles during radioactive decay.
- Half-Life: The half-life of actinium-227, a common isotope in AcCl₃, is about 21.77 years.
- Radiation Safety: Handling this compound requires strict safety protocols due to its radioactivity.
Applications in Science and Medicine
The unique properties of actinium(III) chloride make it useful in several scientific and medical applications.
- Cancer Treatment: Actinium-225, derived from AcCl₃, is used in targeted alpha therapy for cancer treatment.
- Radiopharmaceuticals: It is a key component in developing radiopharmaceuticals for medical imaging and therapy.
- Research Tool: Scientists use actinium(III) chloride in research to study radioactive decay and nuclear reactions.
Production and Synthesis
Producing actinium(III) chloride involves specific methods and processes.
- Extraction: Actinium is extracted from uranium ores as a byproduct.
- Chemical Reaction: Actinium reacts with chlorine gas to form actinium(III) chloride.
- Purification: The compound undergoes purification to remove impurities and achieve high purity levels.
Chemical Reactions
Actinium(III) chloride participates in various chemical reactions, showcasing its reactivity.
- Hydrolysis: It hydrolyzes in water, forming actinium hydroxide and hydrochloric acid.
- Complex Formation: Actinium(III) chloride can form complexes with other ligands, enhancing its chemical versatility.
- Redox Reactions: It can undergo redox reactions, changing its oxidation state under specific conditions.
Safety and Handling
Due to its radioactive nature, handling actinium(III) chloride requires caution.
- Protective Gear: Handling requires protective gear, including gloves and lab coats.
- Storage: It must be stored in lead-lined containers to shield from radiation.
- Disposal: Disposal of actinium(III) chloride follows strict regulations to prevent environmental contamination.
Environmental Impact
The environmental impact of actinium(III) chloride is a concern due to its radioactivity.
- Radioactive Waste: Improper disposal can lead to radioactive contamination of soil and water.
- Containment: Measures are in place to contain and manage radioactive waste from this compound.
- Regulations: Environmental agencies regulate the use and disposal of actinium(III) chloride to protect ecosystems.
Interesting Facts
Here are some additional intriguing facts about actinium(III) chloride.
- Luminescence: Actinium compounds, including AcCl₃, can exhibit luminescence under certain conditions.
- Research Interest: Ongoing research aims to explore new applications and properties of actinium(III) chloride.
- Scarcity: Actinium is a rare element, making its compounds, including AcCl₃, relatively scarce and valuable.
Final Thoughts on Actinium(III) Chloride
Actinium(III) chloride, a compound with the formula AcCl3, holds a unique place in the world of chemistry. Known for its radioactive properties, it’s used in research and potential medical applications. This compound is highly reactive and must be handled with care due to its radioactivity. Actinium itself was discovered in 1899 by Friedrich Oskar Giesel and has since intrigued scientists with its potential uses.
Understanding the properties and applications of actinium(III) chloride can shed light on the broader field of radioactive elements. Its role in cancer treatment research highlights its importance in medical advancements. While not commonly encountered in everyday life, actinium(III) chloride remains a significant subject of study for those interested in nuclear chemistry and medical research. This compound's unique characteristics make it a fascinating topic for further exploration and discovery.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


