Dichlorine tetroxide, also known as chlorine perchlorate, is a fascinating chemical compound with some unique properties. This compound, with the formula Cl2O4, is a yellowish liquid at room temperature. It’s known for being highly reactive and quite unstable, making it a subject of interest for chemists and researchers. Dichlorine tetroxide is used in various industrial applications, including as an oxidizing agent in rocket propellants. However, it’s also highly toxic and corrosive, requiring careful handling and storage. Understanding the properties and uses of this compound can provide insight into its significance in both scientific research and practical applications. Here are 20 intriguing facts about dichlorine tetroxide that will give you a deeper appreciation of this powerful chemical.
Key Takeaways:
- Dichlorine tetroxide, or chlorine perchlorate, is a yellowish liquid with a strong smell. It's used as an oxidizing agent, bleaching agent, and disinfectant, but requires careful handling to avoid environmental impact.
- When working with dichlorine tetroxide, safety measures like protective gear and proper storage are crucial. Its reactivity can harm water, air, and soil, making environmental impact a significant concern.
What is Dichlorine Tetroxide?
Dichlorine tetroxide, also known as chlorine perchlorate, is a chemical compound with some fascinating properties. It’s not something you encounter every day, but it has unique characteristics worth exploring.
-
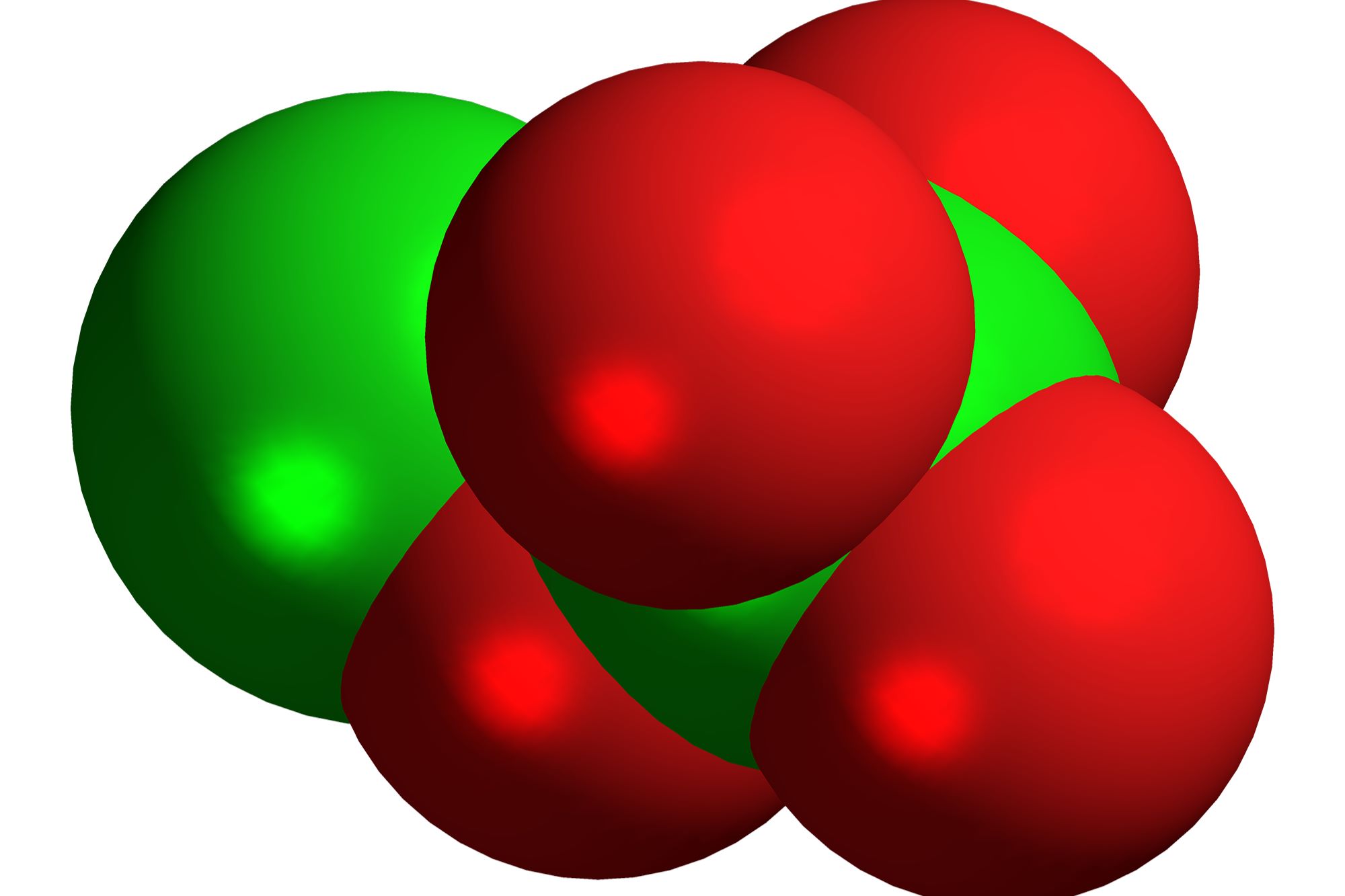
Chemical Formula: Dichlorine tetroxide has the chemical formula Cl2O4. This indicates it contains two chlorine atoms and four oxygen atoms.
-
Appearance: In its pure form, dichlorine tetroxide appears as a yellowish liquid. This color can help identify it in laboratory settings.
-
Odor: It has a strong, pungent smell. This odor is similar to that of chlorine gas, making it easily recognizable.
-
Stability: Dichlorine tetroxide is relatively unstable. It can decompose into chlorine dioxide and chlorine gas, especially when exposed to light or heat.
How is Dichlorine Tetroxide Produced?
The production process of dichlorine tetroxide involves specific chemical reactions. Understanding these methods can provide insight into its properties and uses.
-
Reaction of Chlorine and Ozone: One way to produce dichlorine tetroxide is by reacting chlorine gas with ozone. This method is often used in laboratory settings.
-
Electrolysis: Another production method involves the electrolysis of a mixture of perchloric acid and hydrochloric acid. This process requires careful control of conditions to ensure safety.
Uses of Dichlorine Tetroxide
Despite its instability, dichlorine tetroxide has several applications. These uses take advantage of its unique chemical properties.
-
Oxidizing Agent: Dichlorine tetroxide is a powerful oxidizing agent. It can be used in chemical reactions to add oxygen to other substances.
-
Bleaching Agent: Due to its strong oxidizing properties, it can also serve as a bleaching agent. This application is similar to how chlorine is used in bleaching.
-
Disinfectant: Its ability to kill bacteria and other microorganisms makes it useful as a disinfectant. This property is particularly valuable in water treatment processes.
Safety and Handling of Dichlorine Tetroxide
Handling dichlorine tetroxide requires caution due to its reactive nature. Knowing the safety measures is crucial for anyone working with this compound.
-
Protective Gear: When handling dichlorine tetroxide, wearing protective gear such as gloves, goggles, and lab coats is essential. This gear helps prevent direct contact with the skin and eyes.
-
Ventilation: Working in a well-ventilated area is important. Proper ventilation helps disperse any fumes, reducing the risk of inhalation.
-
Storage: Dichlorine tetroxide should be stored in a cool, dark place. Keeping it away from light and heat sources minimizes the risk of decomposition.
Environmental Impact of Dichlorine Tetroxide
The environmental impact of dichlorine tetroxide is a concern due to its reactive nature. Understanding these effects can help mitigate potential risks.
-
Water Contamination: If released into water sources, dichlorine tetroxide can contaminate them. Its strong oxidizing properties can harm aquatic life.
-
Air Pollution: When decomposed, it releases chlorine gas, a harmful pollutant. This gas can contribute to air quality issues and respiratory problems.
-
Soil Impact: Dichlorine tetroxide can also affect soil quality. Its reactivity can alter the chemical composition of the soil, impacting plant growth.
Interesting Facts about Dichlorine Tetroxide
Beyond its chemical properties and uses, dichlorine tetroxide has some intriguing aspects worth noting.
-
Discovery: Dichlorine tetroxide was first discovered in the 19th century. Its unique properties have intrigued chemists ever since.
-
Research: Ongoing research continues to explore its potential applications. Scientists are particularly interested in its oxidizing abilities.
-
Comparisons: Dichlorine tetroxide is often compared to other chlorine oxides. These comparisons help highlight its unique characteristics.
-
Reactivity: Its high reactivity makes it a subject of study in chemical kinetics. Understanding how it reacts with other substances can provide insights into broader chemical processes.
-
Future Applications: Potential future applications include advanced oxidation processes. These processes could be used in environmental cleanup efforts, showcasing its versatility.
Final Thoughts on Dichlorine Tetroxide
Dichlorine tetroxide, also known as chlorine perchlorate, is a fascinating compound with unique properties. From its chemical structure to its reactivity, this compound plays a significant role in various industrial applications. Its ability to act as an oxidizing agent makes it valuable in chemical synthesis and laboratory research. However, handling it requires caution due to its reactive nature and potential hazards. Understanding these facts can help in appreciating its importance and the precautions needed when working with it. Whether you're a student, researcher, or just curious, knowing about dichlorine tetroxide adds to your knowledge of chemistry and its applications. Stay curious and always prioritize safety when dealing with such compounds.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
