The electron transport chain is a crucial process that occurs in the inner membrane of the mitochondria in eukaryotic cells. It plays a vital role in the production of adenosine triphosphate (ATP), the energy currency of the cell. This remarkable biochemical pathway involves a series of complex reactions that transfer electrons from electron donors to electron acceptors, generating a proton gradient across the membrane.
In this article, we will delve into the fascinating world of the electron transport chain, uncovering 19 captivating facts that shed light on its importance and functionality. From its discovery to its role in cellular respiration, these facts will showcase the incredible intricacies of this fundamental biological process.
Key Takeaways:
- The electron transport chain is like a power plant inside our cells, generating energy in the form of ATP. It’s like a complex energy production line that relies on teamwork and careful regulation to keep our cells running smoothly.
- Just like how a balanced diet and exercise keep our bodies healthy, the electron transport chain also benefits from good nutrition and physical activity. Understanding how it works can help scientists develop new treatments for age-related diseases and mitochondrial disorders.
The electron transport chain is an essential process in cellular respiration.
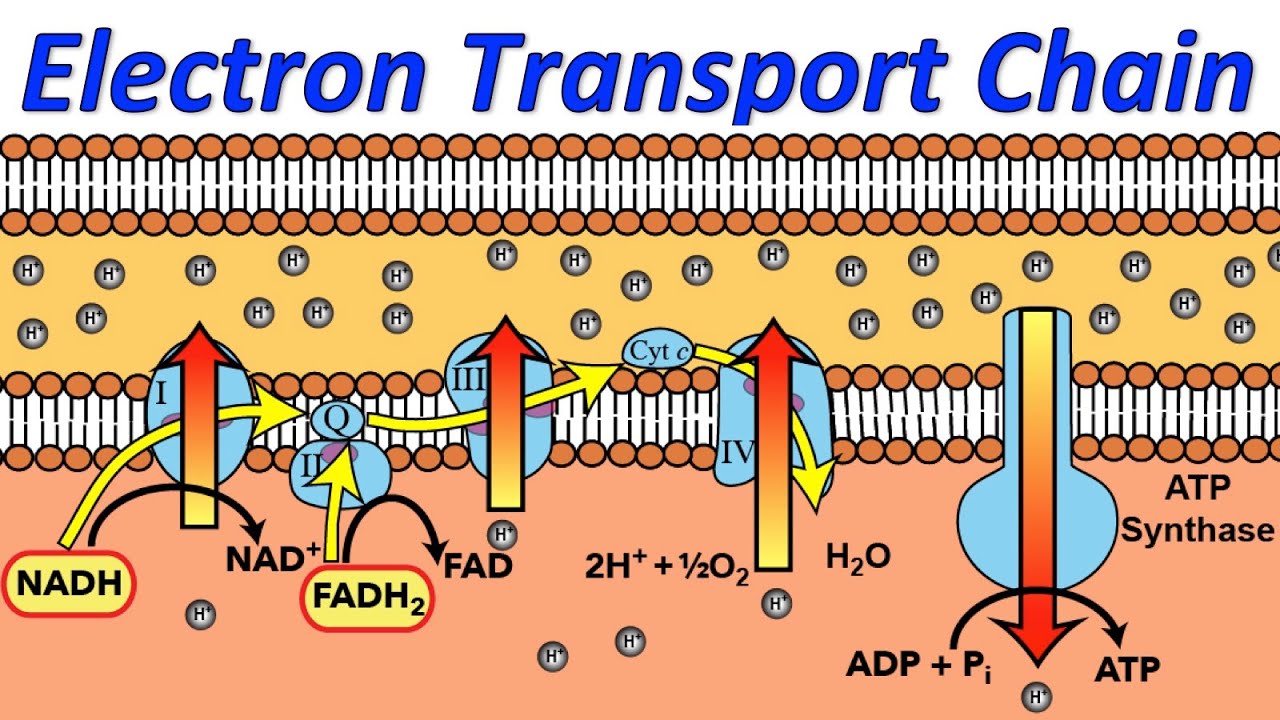
The electron transport chain is a series of protein complexes located in the inner mitochondrial membrane. It plays a crucial role in producing adenosine triphosphate (ATP), the energy currency of cells.
The electron transport chain is composed of four protein complexes.
The four protein complexes involved in the electron transport chain are complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (cytochrome bc1 complex), and complex IV (cytochrome c oxidase).
It relies on electron carriers such as NADH and FADH2.
NADH and FADH2 are high-energy molecules that donate electrons to the electron transport chain, initiating the flow of electrons through the complexes.
Electrons move through the protein complexes in a series of redox reactions.
As electrons pass through the protein complexes, they are transferred from one molecule to another, resulting in a release of energy. This energy is used to pump protons across the mitochondrial membrane.
The electron transport chain creates a proton gradient.
The movement of electrons through the protein complexes pumps protons from the mitochondrial matrix into the intermembrane space, creating a proton gradient.
ATP synthase uses the proton gradient to produce ATP.
The proton gradient generated by the electron transport chain drives ATP synthase to produce ATP. This process is known as oxidative phosphorylation.
The final electron acceptor in the electron transport chain is oxygen.
Oxygen serves as the final electron acceptor in complex IV. It combines with electrons and protons to form water, preventing the accumulation of potentially harmful free radicals.
The electron transport chain is highly efficient at generating ATP.
Compared to other metabolic pathways, the electron transport chain has a high ATP yield. It is estimated that each NADH molecule entering the electron transport chain can produce up to three ATP molecules.
Certain molecules can interfere with the electron transport chain.
Substances such as cyanide and carbon monoxide can disrupt the electron transport chain by binding to cytochrome oxidase, preventing the transfer of electrons to oxygen.
The electron transport chain is a key target of certain antibiotics.
Some antibiotics, such as rotenone and antimycin A, specifically inhibit the electron transport chain in bacteria, making them effective in treating bacterial infections.
The electron transport chain is vital for aerobic organisms.
Aerobic organisms, including humans, rely on the electron transport chain to produce energy in the form of ATP. Without it, cellular respiration cannot occur efficiently.
The electron transport chain generates reactive oxygen species.
During electron transport, some electrons leak and react with molecular oxygen, leading to the production of reactive oxygen species (ROS). ROS can damage cellular components if not properly controlled.
The electron transport chain can be regulated by cellular conditions.
The rate of electron transport can be adjusted depending on the energy demands of the cell. ATP production is regulated by feedback inhibition and the availability of electron carriers.
Mutations in genes encoding electron transport chain proteins can lead to mitochondrial diseases.
Mutations in genes encoding proteins involved in the electron transport chain can result in mitochondrial diseases, which often affect tissues with high energy demands such as the brain and muscles.
The electron transport chain is evolutionarily conserved.
The electron transport chain and oxidative phosphorylation are highly conserved across diverse organisms, suggesting their importance in cellular energy production throughout evolution.
The electron transport chain is a major source of reactive nitrogen species.
In addition to ROS, the electron transport chain can also generate reactive nitrogen species (RNS). These RNS play important roles in cellular signaling and defense mechanisms.
The electron transport chain can be influenced by diet and exercise.
Dietary factors and physical activity can impact the function of the electron transport chain. A balanced diet and regular exercise promote efficient electron transport and ATP production.
The electron transport chain has been studied extensively for its role in aging and age-related diseases.
Research suggests that mitochondrial dysfunction, including impairment of the electron transport chain, plays a significant role in aging and age-related diseases, such as neurodegenerative disorders.
Understanding the electron transport chain can aid in the development of new therapies.
By unraveling the intricacies of the electron transport chain, scientists can identify potential targets for drug development and therapies, particularly in the treatment of mitochondrial disorders and age-related diseases.
Overall, the electron transport chain is a fascinating process that powers cellular respiration and ATP production. Its intricate mechanism and regulatory pathways make it a subject of ongoing research and exploration in the field of biology.
Conclusion
In conclusion, the electron transport chain is a vital process that plays a crucial role in cellular respiration. It is responsible for the production of ATP, the energy currency of cells. Understanding the intricacies of the electron transport chain can provide valuable insights into the functioning of living organisms at a cellular level.Through this article, we have explored 19 fascinating facts about the electron transport chain. We have discovered how electrons are passed through a series of protein complexes and cofactors, generating energy along the way. We have learned about the important role of oxygen as the final electron acceptor and the formation of water as a byproduct.Moreover, we have delved into the significance of the proton gradient and the ATP synthase enzyme in the production of ATP. We have also gained insights into the various inhibitors and uncouplers that can affect the electron transport chain.Overall, the electron transport chain is a remarkable process that showcases the efficiency and complexity of life’s energy conversion mechanisms. It serves as a reminder of the incredible intricacies of the biological world and the wonders that unfold within our cells.
FAQs
1. What is the electron transport chain?
The electron transport chain is a series of protein complexes and electron carriers found in the inner mitochondrial membrane. It plays a crucial role in cellular respiration, transferring electrons and generating ATP.
2. How does the electron transport chain generate ATP?
The electron transport chain generates ATP through a process called oxidative phosphorylation. As electrons pass through the protein complexes, energy is released and used to pump protons across the inner mitochondrial membrane. This creates a proton gradient, which drives the synthesis of ATP by the ATP synthase enzyme.
3. What is the final electron acceptor in the electron transport chain?
Oxygen serves as the final electron acceptor in the electron transport chain. It combines with electrons and protons to form water as a byproduct.
4. Are there any inhibitors of the electron transport chain?
Yes, there are various inhibitors of the electron transport chain, such as rotenone, antimycin A, and cyanide. These substances interfere with the electron transfer process, disrupting ATP production.
5. Can the electron transport chain be uncoupled from ATP synthesis?
Yes, certain compounds known as uncouplers can disrupt the coupling between electron transport and ATP synthesis. Examples include dinitrophenol (DNP) and 2,4-dinitrophenol (DNP), which allow protons to freely flow back into the mitochondrial matrix without generating ATP.
Exploring electron transport chain facts is just the beginning! Delve deeper into this fascinating process with our articles on mindblowing ETC facts, intriguing details about electron transport chain complexes, and captivating insights into photosystem. Each piece offers a unique perspective on these essential components of cellular respiration, shedding light on their roles, mechanisms, and significance in sustaining life. From the intricate workings of protein complexes to the marvels of photosynthesis, these articles will satisfy your curiosity and expand your understanding of the microscopic world within our cells.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
