Centromeric Instability Immunodeficiency, also known as ICF syndrome, is a rare genetic disorder that impacts the immune system and causes unique facial features. This condition, inherited in an autosomal recessive manner, results from mutations in genes like ZBTB24, DNMT3B, CDCA7, and HELLS. Patients often face recurrent infections due to low antibody levels and compromised cellular immunity. Diagnosed typically in early childhood, ICF syndrome also presents with distinct facial anomalies such as wide-set eyes and a flat nasal bridge. Understanding this complex disorder involves exploring its genetic basis, clinical symptoms, diagnostic methods, and treatment options.
Key Takeaways:
- Centromeric Instability Immunodeficiency (ICF) syndrome is a rare genetic disorder affecting the immune system, leading to recurrent infections and distinct facial anomalies. Genetic testing and early diagnosis are crucial for managing this complex condition.
- Understanding the genetic basis, clinical manifestations, and treatment challenges of ICF syndrome is essential for patients and families. Ongoing research aims to improve diagnostic and therapeutic strategies, offering hope for better outcomes in the future.
What is Centromeric Instability Immunodeficiency?
Centromeric Instability Immunodeficiency, also known as ICF syndrome, is a rare genetic disorder that affects the immune system and causes various physical anomalies. Let's dive into the key facts about this condition.
-
ICF Syndrome stands for Immunodeficiency-Centromeric Instability-Facial Anomalies syndrome. It is a rare genetic disorder that affects the immune system and causes various physical anomalies.
-
Genetic Basis: ICF syndrome is caused by mutations in several genes, including ZBTB24, DNMT3B, CDCA7, and HELLS. These genes play crucial roles in DNA methylation and chromosomal stability.
-
Inheritance Pattern: ICF syndrome is inherited in an autosomal recessive manner, meaning that a person must inherit two copies of the mutated gene (one from each parent) to develop the condition.
Clinical Manifestations and Symptoms
Understanding the clinical manifestations and symptoms of ICF syndrome is crucial for early diagnosis and treatment.
-
Recurrent Infections: The primary clinical manifestations of ICF syndrome include recurrent infections, particularly respiratory and gastrointestinal infections.
-
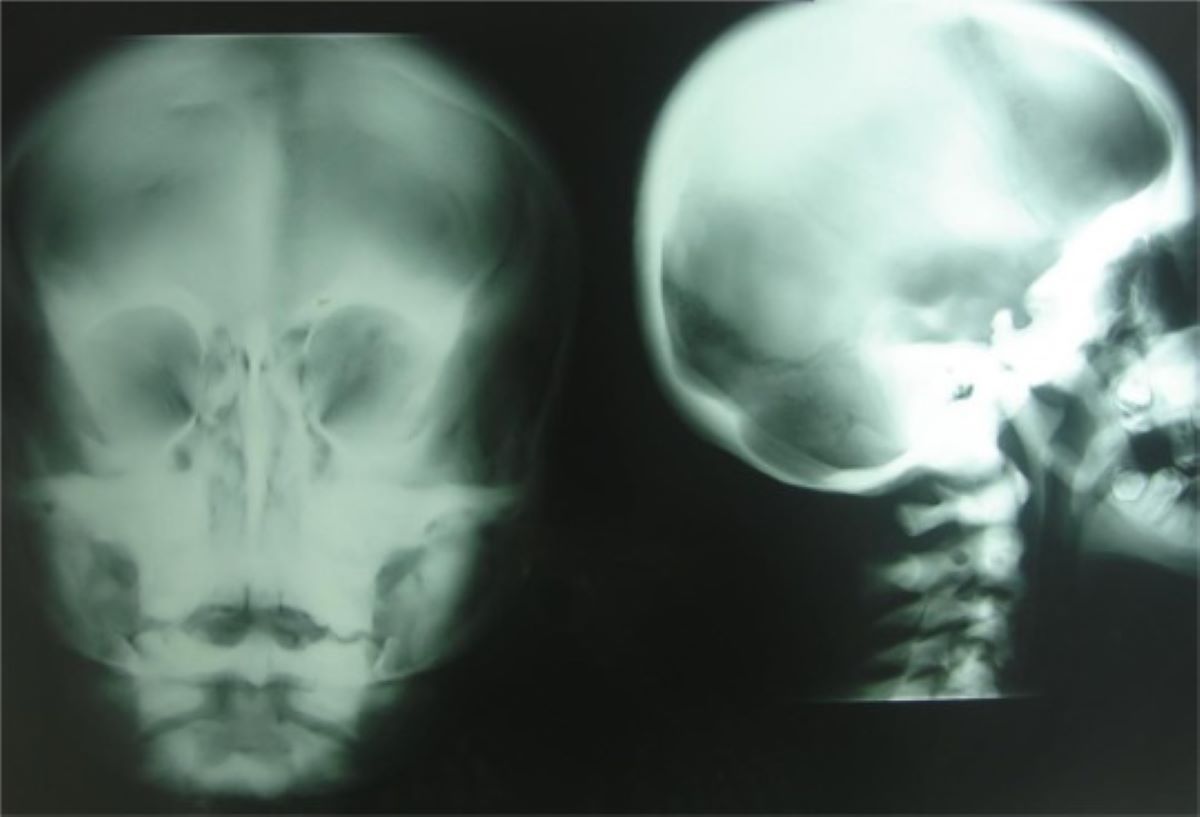
Facial Anomalies: Patients exhibit distinct facial anomalies such as hypertelorism (wide-set eyes), flat nasal bridge, and epicanthus (skin fold at the inner corner of the eye).
-
Immunodeficiency: The hallmark of ICF syndrome is immunodeficiency, characterized by low levels of antibodies (hypogammaglobulinemia) and impaired cellular immunity. This leads to an increased susceptibility to infections.
-
Facial Features: Facial anomalies are a common feature of ICF syndrome. These can include hypertelorism, a flat nasal bridge, and epicanthus. The facial features are often described as bird-like.
Centromeric Instability and Chromosomal Abnormalities
Centromeric instability is a key feature of ICF syndrome, leading to various chromosomal abnormalities.
-
Centromeric Instability: Centromeric instability is a cytogenetic hallmark of ICF syndrome. It involves the juxtacentromeric heterochromatin repeats on chromosomes 1, 9, and 16. This instability can lead to chromosomal abnormalities such as deletions, breaks, and elongation/decondensation in the centromeric region.
-
Chromosomal Abnormalities: Chromosomal analysis in patients with ICF syndrome often reveals deletions, breaks, elongation/decondensation in the centromeric region of chromosomes 1, 16, or 9. Multibranched chromosomes are also a characteristic feature.
Diagnostic Methods and Criteria
Diagnosing ICF syndrome involves a combination of clinical evaluation, chromosomal analysis, and genetic testing.
-
Diagnostic Methods: Diagnosis of ICF syndrome typically involves chromosomal analysis to detect centromeric instability. Additional diagnostic tests include blood tests to assess immunoglobulin levels and lymphocyte count. Genetic testing for mutations in ZBTB24, DNMT3B, CDCA7, and HELLS genes can also confirm the diagnosis.
-
Diagnostic Criteria: The diagnostic criteria for ICF syndrome include recurrent infections, distinct facial anomalies, and centromeric instability detected through chromosomal analysis. Genetic testing for mutations in ZBTB24, DNMT3B, CDCA7, and HELLS genes can confirm the diagnosis.
Genetic Heterogeneity and Prevalence
ICF syndrome exhibits genetic heterogeneity, meaning that mutations in different genes can cause the same condition.
-
Genetic Heterogeneity: ICF syndrome exhibits genetic heterogeneity, meaning that mutations in different genes can cause the same condition. While DNMT3B and ZBTB24 mutations account for a significant portion of cases, other genes like CDCA7 and HELLS are also implicated.
-
Prevalence: ICF syndrome is a rare disorder, with fewer than 70 cases described in the literature. The exact prevalence is difficult to determine due to its rarity and the limited number of reported cases.
Age of Diagnosis and Symptoms in Childhood
ICF syndrome is often diagnosed in early childhood, typically during the first decade of life.
-
Age of Diagnosis: ICF syndrome is often diagnosed in early childhood, typically during the first decade of life. However, some cases have been diagnosed in adulthood, as in the case of a British man who was diagnosed in his 30s.
-
Symptoms in Childhood: Children with ICF syndrome often present with recurrent respiratory and gastrointestinal infections. These infections can be severe and may lead to complications such as bronchiectasis.
Treatment Options and Prognosis
Managing ICF syndrome involves addressing the immunodeficiency and preventing infections.
-
Treatment Options: The primary treatment for ICF syndrome involves immunoglobulin replacement therapy to manage hypogammaglobulinemia. This involves regular intravenous infusions of immunoglobulins to boost antibody levels. In severe cases, hematopoietic stem cell transplantation (HSCT) may be considered to restore immune function.
-
Prognosis: The prognosis for patients with ICF syndrome is generally poor. Most patients die from infections in the first or second decade of life. However, some individuals have survived into adulthood, albeit with significant health challenges.
Role of Specific Genes
Different genes play crucial roles in the development of ICF syndrome, each contributing to the condition's complexity.
-
Role of DNMT3B: Mutations in the DNMT3B gene account for approximately 50% of ICF cases. DNMT3B is a de novo DNA methyltransferase that primarily acts during early development. Mutations in this gene result in reduced methyltransferase activity, leading to hypomethylation of juxtacentromeric satellite types II and III.
-
Role of ZBTB24: Mutations in the ZBTB24 gene account for approximately 30% of ICF cases. The function of ZBTB24 is not fully understood, but it belongs to a family of ZBTB proteins that have regulatory roles in hematopoietic differentiation.
-
Role of CDCA7 and HELLS: Mutations in CDCA7 and HELLS genes have been identified in a subset of unexplained ICF cases. These genes are involved in cell division and chromosomal stability, highlighting the genetic heterogeneity of ICF syndrome.
Cytogenetic Hallmarks and Immune System Dysfunction
Centromeric instability and immune system dysfunction are central to the pathology of ICF syndrome.
-
Cytogenetic Hallmarks: Centromeric instability is the primary cytogenetic hallmark of ICF syndrome. This involves hypomethylation of juxtacentromeric satellite types II and III, as well as centromeric α-satellite repeats in DNMT3B mutation-negative patients.
-
Immune System Dysfunction: ICF syndrome is characterized by dysfunction in both B cell and T cell immunity. This leads to a compromised immune response, making patients susceptible to infections.
Facial Dysmorphism and Recurrent Infections
Facial dysmorphism and recurrent infections are prominent features of ICF syndrome.
-
Facial Dysmorphism: Facial dysmorphism is a common feature of ICF syndrome. The facial anomalies can include hypertelorism, a flat nasal bridge, and epicanthus, which are often described as bird-like.
-
Recurrent Infections: Recurrent infections are the primary presenting symptom of ICF syndrome. These infections can be severe and may lead to complications such as bronchiectasis.
Hematopoietic Stem Cell Transplantation (HSCT) and Future Research
HSCT offers a potential cure for severe cases, while ongoing research aims to better understand the condition.
-
Hematopoietic Stem Cell Transplantation (HSCT): HSCT is a curative treatment option for patients with severe cellular dysfunction associated with ICF syndrome. This procedure involves replacing the bone marrow with healthy stem cells to restore immune function.
-
Future Research Directions: Future research directions for ICF syndrome include further elucidating the pathophysiological mechanisms underlying the condition. Understanding the genetic heterogeneity and the role of different genes involved in ICF syndrome will help in developing more effective diagnostic and therapeutic strategies.
Genetic Counseling and Research Advances
Genetic counseling and recent research advances are crucial for managing ICF syndrome.
-
Genetic Counseling: Genetic counseling is essential for families affected by ICF syndrome. It helps in understanding the inheritance pattern and the risk of passing the mutated genes to offspring.
-
Research Advances: Recent research has advanced our understanding of ICF syndrome by identifying new genes involved in the condition. This knowledge has implications for diagnosis and treatment strategies.
Treatment Challenges and Survival Stories
Despite the challenges, some patients have survived into adulthood, highlighting the variability of ICF syndrome.
-
Treatment Challenges: Treatment of ICF syndrome is challenging due to the complex nature of the condition. Patients often require lifelong immunoglobulin replacement therapy and may need additional treatments for complications such as bronchiectasis.
-
Survival Stories: Despite the poor prognosis, there are instances where patients have survived into adulthood. These cases highlight the variability in the severity and progression of ICF syndrome.
Final Thoughts on ICF Syndrome
ICF syndrome, short for Immunodeficiency-Centromeric Instability-Facial Anomalies syndrome, is a rare genetic disorder that packs a punch. With its roots in mutations of genes like ZBTB24, DNMT3B, CDCA7, and HELLS, this condition leads to serious immunodeficiency, centromeric instability, and distinct facial anomalies. Diagnosed mainly in early childhood, it presents with recurrent infections and unique facial features. Chromosomal analysis and genetic testing are key to pinpointing this disorder. Treatments like immunoglobulin replacement therapy and, in severe cases, hematopoietic stem cell transplantation offer some hope. Despite the challenges, ongoing research is shedding light on better diagnostic and therapeutic strategies. Understanding ICF syndrome is crucial for improving patient outcomes and providing support to affected families.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.