Fick's Laws of Diffusion are fundamental principles in physics and chemistry that describe how particles move from areas of high concentration to low concentration. These laws are essential for understanding processes in fields like biology, engineering, and environmental science. Fick's First Law deals with the rate of diffusion, while Fick's Second Law focuses on how diffusion causes concentration to change over time. Understanding these laws can help explain phenomena such as how oxygen travels through tissues, how pollutants spread in the air, or how flavors mix in cooking. In this post, we'll explore 36 intriguing facts about Fick's Laws of Diffusion to give you a deeper appreciation of their significance and applications.
What are Fick's Laws of Diffusion?
Fick's Laws of Diffusion describe how particles move from areas of high concentration to low concentration. These laws are fundamental in fields like chemistry, biology, and physics.
- First Law: This law states that the flux of particles is proportional to the concentration gradient. In simpler terms, particles move faster when there's a bigger difference in concentration.
- Second Law: This law predicts how diffusion causes the concentration to change over time. It helps scientists understand how substances spread out in a medium.
History of Fick's Laws
Understanding the background of these laws can give more context to their importance.
- Adolf Fick: A German physiologist named Adolf Fick formulated these laws in 1855.
- Inspiration: Fick was inspired by Fourier's work on heat conduction, which has similar mathematical principles.
- First Publication: Fick's original paper was titled "On Liquid Diffusion" and was published in the journal "Annalen der Physik."
Applications in Science
Fick's Laws are not just theoretical; they have practical applications in various scientific fields.
- Biology: These laws help explain how oxygen and nutrients diffuse through cell membranes.
- Chemistry: They are used to understand how chemicals mix in solutions.
- Physics: In physics, these laws describe how particles spread in different states of matter.
- Medicine: Drug delivery systems often rely on these principles to ensure proper dosage and distribution.
Mathematical Formulation
The math behind Fick's Laws can be complex, but it's crucial for accurate predictions.
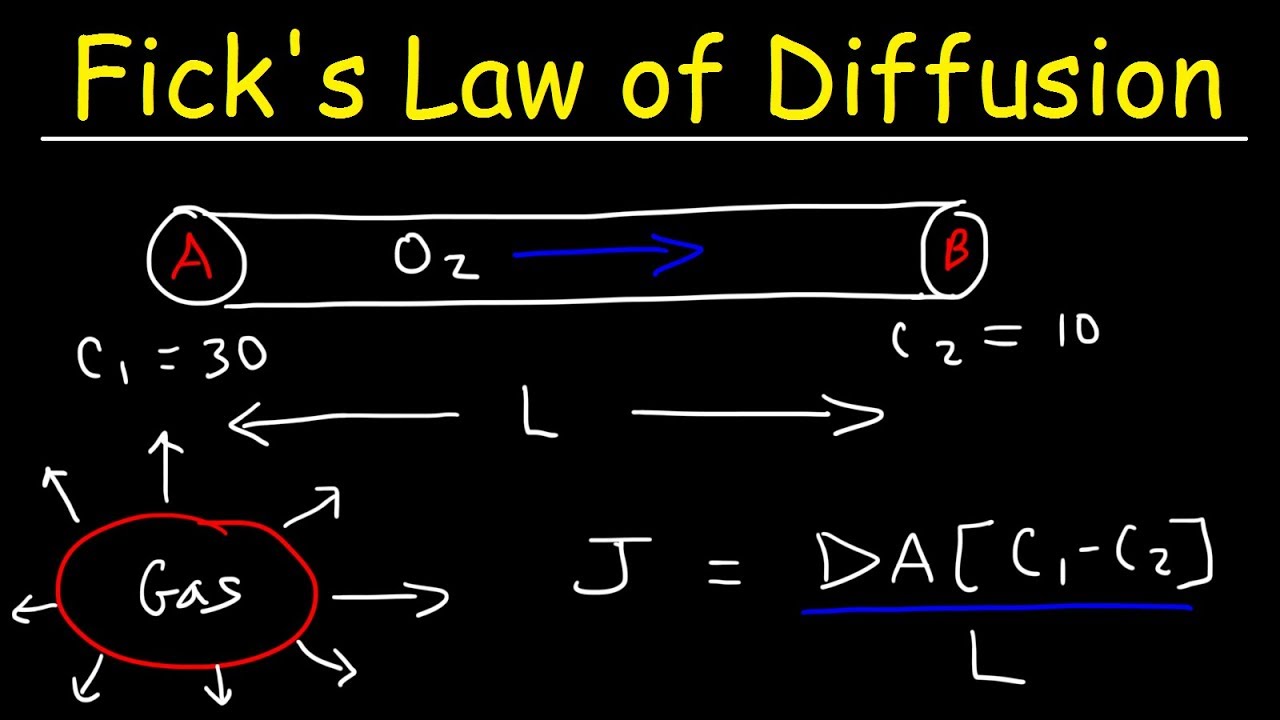
- First Law Equation: The first law is often written as J = -D(dC/dx), where J is the flux, D is the diffusion coefficient, and dC/dx is the concentration gradient.
- Second Law Equation: The second law is expressed as ∂C/∂t = D∂²C/∂x², where ∂C/∂t is the rate of change of concentration over time.
- Diffusion Coefficient: The diffusion coefficient (D) varies depending on the medium and the substance being diffused.
Real-World Examples
Seeing how these laws apply in real life can make them easier to understand.
- Perfume: When you spray perfume, the scent spreads through the air due to diffusion.
- Tea: When you put a tea bag in hot water, the tea diffuses out into the water.
- Ink in Water: Dropping ink into water shows how the ink particles spread out over time.
Factors Affecting Diffusion
Several factors can influence how quickly or slowly diffusion occurs.
- Temperature: Higher temperatures increase the energy of particles, speeding up diffusion.
- Medium: Diffusion happens faster in gases than in liquids and slower in solids.
- Particle Size: Smaller particles diffuse more quickly than larger ones.
- Concentration Gradient: A steeper gradient results in faster diffusion.
Advanced Concepts
For those interested in diving deeper, there are more advanced topics related to Fick's Laws.
- Non-Steady State Diffusion: This occurs when the concentration gradient changes over time, requiring more complex calculations.
- Multicomponent Diffusion: Involves more than one type of particle, making the math even more intricate.
- Anomalous Diffusion: Sometimes, diffusion doesn't follow Fick's Laws exactly, leading to "anomalous" behavior.
Fick's Laws in Modern Research
These laws continue to be relevant in cutting-edge scientific research.
- Nanotechnology: Understanding diffusion at the nanoscale is crucial for developing new materials.
- Environmental Science: These principles help model the spread of pollutants in air and water.
- Astrophysics: Diffusion processes are studied to understand the behavior of gases in space.
Fun Facts
Here are some interesting tidbits about Fick's Laws and diffusion.
- Brownian Motion: The random movement of particles suspended in a fluid, known as Brownian motion, is a form of diffusion.
- Einstein's Contribution: Albert Einstein expanded on Fick's work to explain Brownian motion mathematically.
- Diffusion in Cooking: Marinating meat relies on diffusion to spread flavors throughout the food.
Challenges and Limitations
Despite their usefulness, Fick's Laws have some limitations.
- Assumptions: These laws assume a uniform medium, which isn't always the case in real-world scenarios.
- Complex Systems: In highly complex systems, other forces can affect particle movement, making Fick's Laws less accurate.
- Scale: At very small scales, quantum effects can alter diffusion behavior.
Educational Importance
Learning about Fick's Laws can be beneficial for students and educators alike.
- Curriculum: These laws are often included in high school and college science curricula.
- Experiments: Simple experiments, like observing food coloring in water, can help students grasp the concept.
- Interdisciplinary: Understanding these principles can aid in learning other scientific concepts, like osmosis and heat transfer.
Future Prospects
The study of diffusion continues to evolve, opening new avenues for research.
- Artificial Intelligence: AI algorithms are being developed to model diffusion processes more accurately.
- Medical Advances: Improved understanding of diffusion could lead to better drug delivery systems and treatments.
That's a wrap on Fick's Laws of Diffusion.
The Final Word on Fick's Laws
Fick's Laws of Diffusion are essential for understanding how substances move. These principles apply to many fields, from biology to engineering. Knowing how particles spread can help in designing better medical treatments, improving industrial processes, and even understanding environmental changes.
Fick's First Law deals with steady-state diffusion, where the concentration gradient remains constant. Fick's Second Law addresses non-steady-state diffusion, where the concentration changes over time. Both laws are crucial for predicting how substances will behave in different scenarios.
Incorporating these laws into practical applications can lead to more efficient systems and innovative solutions. Whether you're a student, a professional, or just curious, grasping these concepts can open up new perspectives.
So, next time you encounter a diffusion problem, remember Fick's Laws. They might just provide the clarity you need.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
