What is the Franck-Condon Principle? The Franck-Condon Principle explains why certain electronic transitions in molecules are more likely than others. It states that during an electronic transition, the positions of the nuclei in a molecule remain unchanged because electrons move much faster than nuclei. This principle helps predict the intensity of spectral lines in molecular spectroscopy. Named after James Franck and Edward Condon, it plays a crucial role in understanding molecular vibrations and electronic states. Whether you're a chemistry enthusiast or just curious about how molecules behave, this principle offers fascinating insights into the quantum world.
What is the Franck-Condon Principle?
The Franck-Condon Principle is a concept in quantum mechanics and spectroscopy. It explains the intensity distribution of electronic transitions in molecules. Named after James Franck and Edward Condon, this principle is crucial for understanding molecular spectroscopy.
-
The Franck-Condon Principle states that electronic transitions occur so quickly that the nuclei of the molecules do not move during the transition.
-
This principle helps predict the intensity of spectral lines in electronic transitions.
-
It is based on the Born-Oppenheimer approximation, which separates electronic and nuclear motions in molecules.
-
The principle is often visualized using potential energy curves for the ground and excited states of a molecule.
Historical Background
Understanding the history behind the Franck-Condon Principle can provide context for its importance in modern science.
-
James Franck and Edward Condon independently proposed the principle in the 1920s.
-
Franck received the Nobel Prize in Physics in 1925 for his work on the excitation and ionization of atoms by electron impact.
-
Edward Condon contributed significantly to quantum mechanics and theoretical physics, including the development of the Franck-Condon Principle.
-
The principle was initially used to explain the absorption spectra of diatomic molecules.
Applications in Spectroscopy
The Franck-Condon Principle has numerous applications in the field of spectroscopy, making it a vital tool for scientists.
-
It helps in understanding the vibrational structure of electronic spectra.
-
The principle is used in the analysis of fluorescence and phosphorescence spectra.
-
It aids in the interpretation of photoelectron spectra, which provide information about the electronic structure of molecules.
-
The principle is essential for studying the dynamics of photochemical reactions.
Quantum Mechanical Basis
The Franck-Condon Principle is deeply rooted in quantum mechanics, providing a theoretical framework for its application.
-
It relies on the overlap of vibrational wavefunctions in the ground and excited states.
-
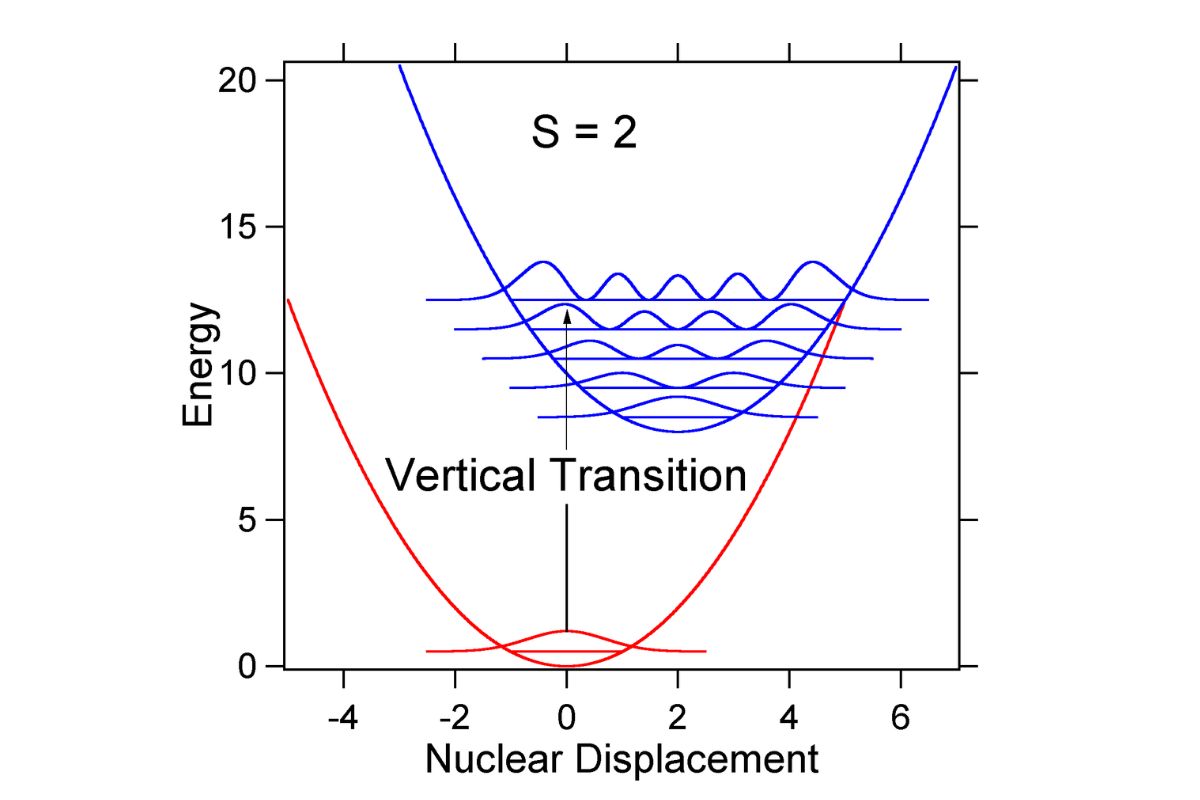
The principle uses the concept of vertical transitions, where the electronic transition occurs without a change in the nuclear configuration.
-
The Franck-Condon factors, which are the overlap integrals of vibrational wavefunctions, determine the intensity of spectral lines.
-
These factors are crucial for calculating the probability of electronic transitions.
Visualization and Diagrams
Visual aids like potential energy diagrams help in understanding the Franck-Condon Principle better.
-
Potential energy curves illustrate the energy levels of molecules in different electronic states.
-
The vertical transitions between these curves represent the electronic transitions predicted by the Franck-Condon Principle.
-
These diagrams help in visualizing the overlap of vibrational wavefunctions.
-
They are used to explain the intensity distribution of spectral lines in electronic transitions.
Importance in Molecular Physics
The Franck-Condon Principle plays a significant role in the field of molecular physics, influencing various research areas.
-
It helps in understanding the photophysics of molecules.
-
The principle is used in the study of molecular dynamics and reaction mechanisms.
-
It aids in the development of new materials with specific optical properties.
-
The principle is essential for interpreting experimental data in molecular spectroscopy.
Real-World Examples
Real-world examples help illustrate the practical applications of the Franck-Condon Principle.
-
The principle is used in the design of organic light-emitting diodes (OLEDs).
-
It helps in the development of solar cells by understanding the electronic transitions in photovoltaic materials.
-
The principle is applied in the study of biological molecules, such as proteins and DNA.
-
It aids in the analysis of environmental pollutants through spectroscopic techniques.
Challenges and Limitations
Despite its importance, the Franck-Condon Principle has certain limitations and challenges.
-
It assumes that electronic transitions are instantaneous, which may not always be accurate.
-
The principle does not account for non-vertical transitions, which can occur in some cases.
-
It may not be applicable to complex molecules with multiple electronic states.
-
The principle requires accurate potential energy curves, which can be difficult to obtain for some molecules.
Future Directions
Research continues to expand the applications and understanding of the Franck-Condon Principle.
-
Advances in computational chemistry are improving the accuracy of potential energy curves.
-
New spectroscopic techniques are being developed to study non-vertical transitions and complex molecules.
The Franck-Condon Principle in a Nutshell
The Franck-Condon Principle is a cornerstone in understanding molecular spectroscopy. It explains why electronic transitions in molecules often occur without significant changes in nuclear positions. This principle helps predict the intensity of spectral lines, making it crucial for interpreting absorption and emission spectra. By considering the overlap of vibrational wavefunctions, scientists can better understand molecular behavior during electronic transitions. This knowledge is applied in fields like chemistry, physics, and materials science. Whether you're a student or a seasoned researcher, grasping the Franck-Condon Principle can deepen your appreciation for the intricate dance of electrons and nuclei in molecules. So next time you look at a spectrum, remember the invisible yet powerful rules guiding those peaks and valleys.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
