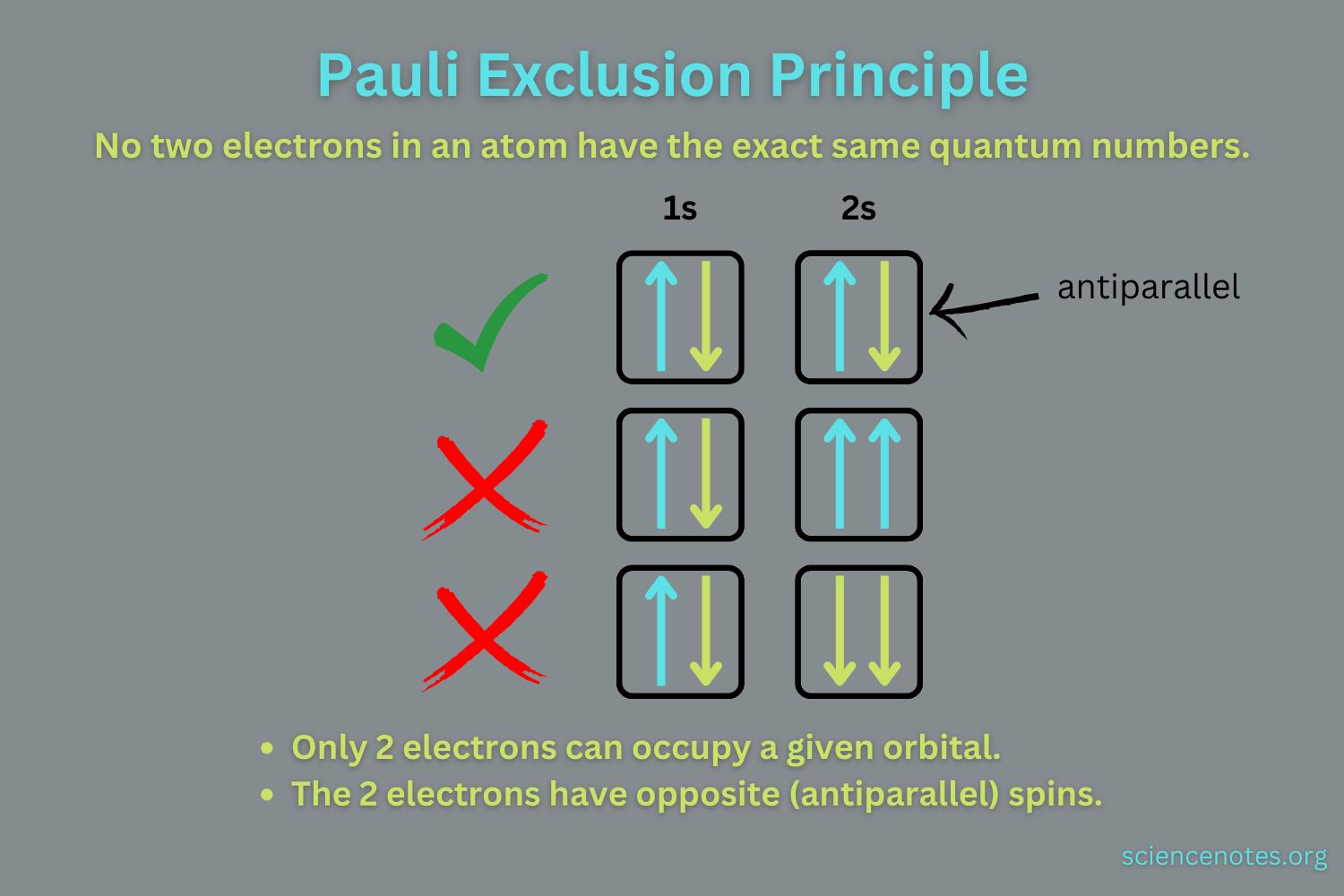
What is the Pauli Exclusion Principle? The Pauli Exclusion Principle is a fundamental concept in quantum mechanics. It states that no two fermions (particles like electrons, protons, and neutrons) can occupy the same quantum state simultaneously. This principle, formulated by Wolfgang Pauli in 1925, explains why electrons in an atom fill up different energy levels or orbitals. Without this rule, all electrons would collapse into the lowest energy state, making the universe vastly different. The principle is crucial for understanding the structure of atoms, the behavior of particles in a star, and even the properties of materials. In essence, it helps explain why matter has structure and stability.
What is the Pauli Exclusion Principle?
The Pauli Exclusion Principle is a fundamental concept in quantum mechanics. It states that no two identical fermions (particles with half-integer spin) can occupy the same quantum state simultaneously. This principle has profound implications in physics, chemistry, and even the structure of the universe.
-
Named After Wolfgang Pauli: The principle is named after Austrian physicist Wolfgang Pauli, who formulated it in 1925.
-
Applies to Fermions: This principle specifically applies to fermions, which include particles like electrons, protons, and neutrons.
-
Quantum State: A quantum state is defined by a set of quantum numbers. For electrons in an atom, these include the principal, angular momentum, magnetic, and spin quantum numbers.
Importance in Atomic Structure
The Pauli Exclusion Principle plays a crucial role in determining the structure of atoms and the periodic table.
-
Electron Configuration: It explains why electrons fill orbitals in a specific order, leading to the unique electron configurations of elements.
-
Periodic Table: The arrangement of elements in the periodic table is a direct consequence of this principle, dictating the chemical properties of elements.
-
Stability of Matter: Without this principle, electrons would collapse into the lowest energy state, making stable matter impossible.
Role in Chemistry
In chemistry, the Pauli Exclusion Principle helps explain the behavior of atoms in molecules and chemical reactions.
-
Chemical Bonds: It explains why atoms form specific types of bonds, such as covalent and ionic bonds.
-
Molecular Orbitals: The principle governs the filling of molecular orbitals, affecting the shape and stability of molecules.
-
Valence Electrons: It determines the number of valence electrons, which are crucial for chemical reactivity.
Applications in Physics
Beyond chemistry, the Pauli Exclusion Principle has significant implications in various fields of physics.
-
Solid-State Physics: It explains the electronic properties of solids, including conductors, semiconductors, and insulators.
-
Astrophysics: The principle is essential in understanding the structure of white dwarfs and neutron stars.
-
Quantum Computing: It plays a role in the development of quantum computers, which rely on the principles of quantum mechanics.
Interesting Facts
Here are some intriguing facts about the Pauli Exclusion Principle that highlight its importance and applications.
-
Pauli's Nobel Prize: Wolfgang Pauli received the Nobel Prize in Physics in 1945 for his discovery of the exclusion principle.
-
Electron Shells: The principle explains why electron shells fill up in a specific order, leading to the unique properties of each element.
-
Magnetic Properties: It influences the magnetic properties of materials, including ferromagnetism and antiferromagnetism.
-
Superfluidity: The principle is crucial in explaining superfluidity, a phase of matter with zero viscosity.
-
Stellar Evolution: It plays a role in the life cycle of stars, particularly in the formation of white dwarfs and neutron stars.
-
Quantum Numbers: The principle requires that all four quantum numbers for any two electrons in an atom must be different.
-
Electron Degeneracy Pressure: This pressure, arising from the Pauli Exclusion Principle, prevents white dwarfs from collapsing under gravity.
-
Neutron Stars: In neutron stars, neutron degeneracy pressure, similar to electron degeneracy pressure, prevents collapse.
-
Chemical Periodicity: The principle explains the periodicity observed in the periodic table, leading to the recurring chemical properties of elements.
-
Spectral Lines: It helps explain the spectral lines of elements, which are unique to each element due to their electron configurations.
-
Atomic Orbitals: The principle dictates the shape and orientation of atomic orbitals, influencing the geometry of molecules.
-
Spin-Statistics Theorem: This theorem, related to the Pauli Exclusion Principle, states that particles with half-integer spin are fermions, while those with integer spin are bosons.
-
Quantum Entanglement: The principle plays a role in quantum entanglement, a phenomenon where particles become interconnected and the state of one affects the other.
-
Laser Technology: It is fundamental in the operation of lasers, which rely on the principles of quantum mechanics.
-
Magnetic Resonance Imaging (MRI): The principle is used in MRI technology, which relies on the magnetic properties of atomic nuclei.
-
Chemical Reactivity: It influences the reactivity of elements, determining how they interact with other substances.
-
Nuclear Structure: The principle helps explain the structure of atomic nuclei, including the arrangement of protons and neutrons.
-
Quantum Field Theory: It is a cornerstone of quantum field theory, which describes the behavior of fundamental particles and forces.
Final Thoughts on Pauli Exclusion Principle
The Pauli Exclusion Principle is a cornerstone of quantum mechanics. It explains why electrons in an atom occupy different energy levels, preventing them from collapsing into a single state. This principle not only shapes the structure of atoms but also influences the properties of matter, from the stability of white dwarfs to the behavior of metals. Understanding this principle helps us grasp the complexities of the microscopic world. It’s fascinating how a rule about particles not sharing the same quantum state can have such a profound impact on the universe. Next time you look at the periodic table or marvel at the stars, remember the Pauli Exclusion Principle is at work. This principle is a testament to the intricate and beautiful laws governing our universe.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
