Molecular Orbital Theory is a fundamental concept in chemistry that explains how atoms combine to form molecules. But what exactly is Molecular Orbital Theory? In simple terms, it describes the behavior of electrons in a molecule, treating them as if they occupy orbitals that belong to the entire molecule rather than individual atoms. This theory helps predict the magnetic and spectral properties of molecules, their stability, and how they interact with light. Understanding this theory can be a game-changer for anyone studying chemistry, as it provides a deeper insight into the nature of chemical bonds. Ready to dive into some fascinating facts about Molecular Orbital Theory? Let's get started!
What is Molecular Orbital Theory?
Molecular Orbital Theory (MOT) is a fundamental concept in chemistry that explains how atoms combine to form molecules. It describes the behavior of electrons in a molecule in terms of molecular orbitals, which are formed by the combination of atomic orbitals. Here are some intriguing facts about this theory:
-
Developed in the 1930s: Molecular Orbital Theory was developed by Robert S. Mulliken and Friedrich Hund in the early 1930s. Their work laid the foundation for modern quantum chemistry.
-
Electrons in Molecules: Unlike the Valence Bond Theory, which considers electrons to be localized between atoms, MOT treats electrons as delocalized over the entire molecule.
-
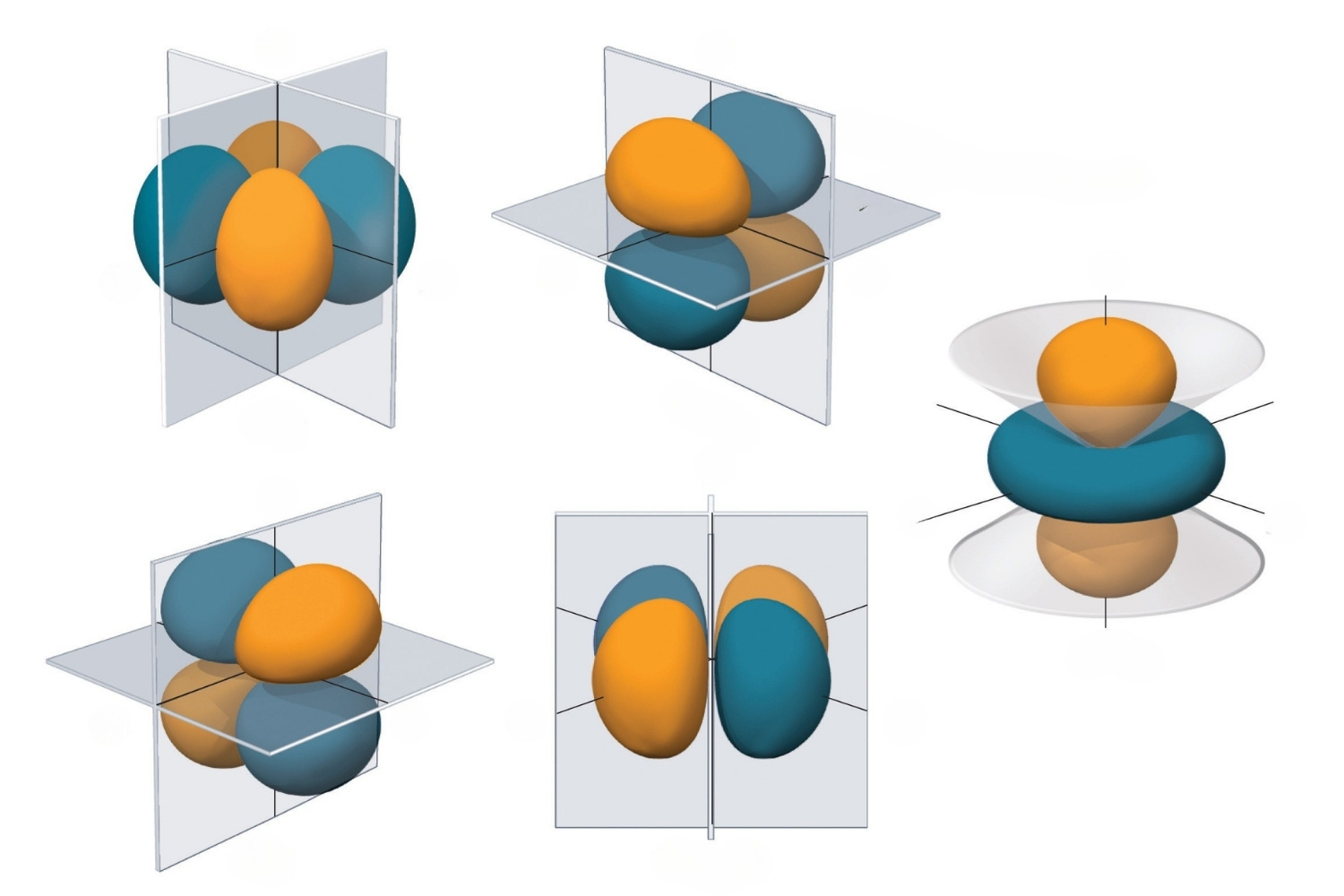
Bonding and Antibonding Orbitals: When atomic orbitals combine, they form bonding and antibonding molecular orbitals. Bonding orbitals lower the energy of the molecule, while antibonding orbitals increase it.
-
Sigma and Pi Bonds: Molecular orbitals can form sigma (σ) and pi (π) bonds. Sigma bonds are formed by the head-on overlap of orbitals, while pi bonds result from the side-by-side overlap.
-
HOMO and LUMO: The Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO) are crucial in determining a molecule's reactivity. The energy gap between them is called the band gap.
-
Electron Configuration: Just like atoms, molecules have electron configurations. Electrons fill molecular orbitals starting from the lowest energy level, following the Pauli exclusion principle and Hund's rule.
Applications of Molecular Orbital Theory
MOT isn't just a theoretical concept; it has practical applications in various fields of chemistry and materials science. Here are some examples:
-
Predicting Molecular Stability: By analyzing the molecular orbitals, chemists can predict the stability of a molecule. A molecule is stable if it has more electrons in bonding orbitals than in antibonding orbitals.
-
Spectroscopy: Molecular Orbital Theory helps explain the absorption and emission spectra of molecules. Transitions between molecular orbitals correspond to specific wavelengths of light.
-
Chemical Reactions: MOT provides insights into the mechanisms of chemical reactions. It helps predict which bonds will break and form during a reaction.
-
Magnetism: The theory can explain the magnetic properties of molecules. Molecules with unpaired electrons in their molecular orbitals exhibit paramagnetism.
-
Photochemistry: In photochemistry, MOT helps understand how light interacts with molecules, leading to excited states and subsequent chemical reactions.
-
Material Science: Molecular Orbital Theory is used to design new materials with specific properties, such as semiconductors and superconductors.
Key Concepts in Molecular Orbital Theory
Several key concepts form the backbone of Molecular Orbital Theory. Understanding these concepts is essential for grasping the theory's full scope.
-
Linear Combination of Atomic Orbitals (LCAO): Molecular orbitals are formed by the linear combination of atomic orbitals. This method approximates the molecular orbitals as a sum of atomic orbitals.
-
Symmetry of Orbitals: The symmetry of atomic orbitals plays a crucial role in their combination. Only orbitals with compatible symmetries can combine to form molecular orbitals.
-
Overlap Integral: The overlap integral measures the extent of overlap between atomic orbitals. A higher overlap integral indicates a stronger interaction and a more stable bonding orbital.
-
Orbital Mixing: In some cases, atomic orbitals of different energies can mix, leading to new molecular orbitals. This phenomenon is known as orbital mixing or hybridization.
-
Molecular Orbital Diagrams: These diagrams visually represent the energy levels of molecular orbitals. They help chemists understand the distribution of electrons in a molecule.
-
Bond Order: Bond order is a measure of the number of chemical bonds between a pair of atoms. It is calculated as the difference between the number of electrons in bonding and antibonding orbitals divided by two.
Advanced Topics in Molecular Orbital Theory
For those who want to delve deeper, here are some advanced topics related to Molecular Orbital Theory.
-
Density Functional Theory (DFT): DFT is a computational quantum mechanical modeling method used to investigate the electronic structure of molecules. It is based on the principles of Molecular Orbital Theory.
-
Extended Hückel Theory: This is an approximation method used to calculate the electronic structure of molecules. It extends the Hückel method to include non-π electrons.
-
Molecular Orbital Perturbation Theory: This theory deals with the changes in molecular orbitals due to external perturbations, such as electric fields or interactions with other molecules.
-
Frontier Molecular Orbital Theory: This theory focuses on the interactions between the HOMO of one molecule and the LUMO of another. It is particularly useful in understanding chemical reactivity and selectivity.
-
Photoelectron Spectroscopy: This technique measures the energy of electrons ejected from a molecule by photons. The results provide information about the molecular orbitals and their energies.
-
Computational Chemistry: Molecular Orbital Theory is a cornerstone of computational chemistry. It allows scientists to simulate and predict the behavior of molecules using computer algorithms.
Fun Facts About Molecular Orbital Theory
Molecular Orbital Theory isn't just for scientists; it has some fun and surprising aspects too.
-
Nobel Prize: Robert S. Mulliken received the Nobel Prize in Chemistry in 1966 for his work on Molecular Orbital Theory.
-
Color of Compounds: The theory helps explain why certain compounds have specific colors. The color arises from electronic transitions between molecular orbitals.
-
Aromaticity: Molecular Orbital Theory explains the concept of aromaticity in organic chemistry. Aromatic compounds have a ring of atoms with delocalized π-electrons, leading to exceptional stability.
The Final Word on Molecular Orbital Theory
Molecular Orbital Theory isn't just for scientists in labs. It helps us understand how atoms bond to form molecules, which is crucial for everything from creating new materials to understanding biological processes. By knowing how electrons are distributed in molecules, we can predict properties like magnetism, color, and reactivity. This theory also bridges the gap between quantum mechanics and chemistry, making it a cornerstone of modern science. Whether you're a student, a researcher, or just curious, grasping the basics of Molecular Orbital Theory can open up a whole new world of understanding. So next time you look at a molecule, remember there's a lot more going on than meets the eye. Keep exploring, keep questioning, and let the wonders of chemistry inspire you.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
