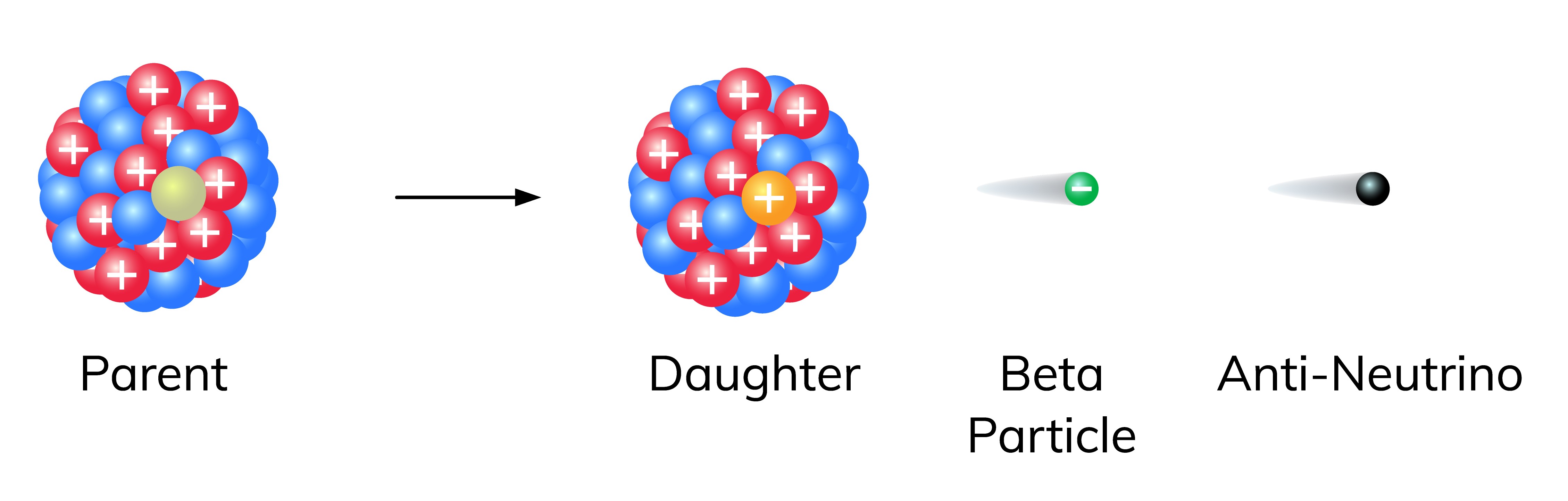
Beta decay is a fascinating phenomenon in the field of physics that involves the transformation of atomic nuclei. It is a type of radioactive decay in which a nucleus emits or captures a beta particle, resulting in the conversion of a neutron into a proton or vice versa. This process is governed by the weak nuclear force, one of the four fundamental forces of nature.
In this article, we will delve into the incredible world of beta decay and explore ten extraordinary facts about this intriguing phenomenon. From its discovery to its role in understanding the composition of matter, beta decay has revolutionized our understanding of the universe. So, buckle up and get ready to uncover some mind-blowing insights into the world of beta decay!
Key Takeaways:
- Beta decay is a nuclear process that changes elements and helps in medical treatments. It’s like a tiny atomic makeover that scientists can use to understand and improve our world.
- Beta decay follows a special law and helps keep atomic nuclei stable. It’s like nature’s way of balancing the number of neutrons and protons, kind of like a tiny nuclear dance to keep things in harmony.
Beta Decay is a Nuclear Process
Beta decay is a nuclear process that occurs in certain radioactive elements. It involves the transformation of a neutron or a proton within an atomic nucleus into a beta particle (either an electron or a positron) and a corresponding neutrino or antineutrino.
Beta Decay is a Form of Radioactive Decay
Beta decay is one of the three primary forms of radioactive decay, alongside alpha decay and gamma decay. It occurs in isotopes that have an excess of either neutrons or protons in their nuclei, leading to the conversion of a neutron to a proton or a proton to a neutron.
Beta Decay Can Occur in Two Forms
Beta decay can occur in two different forms: beta-minus (?-) decay and beta-plus (?+) decay. In beta-minus decay, a neutron within the nucleus is converted into a proton, emitting an electron and an electron antineutrino. In beta-plus decay, a proton is transformed into a neutron, releasing a positron and an electron neutrino.
Beta Decay Leads to Element Transmutations
As a result of beta decay, the number of protons in the nucleus changes, leading to the transformation of one element into another. This process is crucial in the natural decay of radioactive isotopes and plays a significant role in the formation of elements and their isotopes in the universe.
Beta Decay Is Governed by the Weak Nuclear Force
The weak nuclear force, one of the four fundamental forces of nature, governs the process of beta decay. This force is responsible for the transformation of a neutron or a proton within the atomic nucleus and the emission of beta particles and neutrinos.
Beta Decay Plays a Role in Nuclear Medicine
Beta decay is utilized in nuclear medicine for various diagnostic and therapeutic purposes. Radioactive isotopes that undergo beta decay are often used to image and treat certain medical conditions, such as cancer.
Beta Decay Can Be Influenced by External Factors
External factors such as temperature, pressure, and electromagnetic fields can influence the rate of beta decay. By altering these conditions, scientists can manipulate the decay rate and better understand the underlying processes involved.
Beta Decay Is Essential for Carbon Dating
Beta decay is essential in the technique of carbon dating, which is used to determine the age of organic materials. By measuring the ratio of carbon-14 (which undergoes beta decay) to carbon-12 in a sample, scientists can estimate its age.
Beta Decay Follows an Exponential Decay Law
The rate of beta decay follows an exponential decay law, meaning that the decay rate decreases exponentially over time. This characteristic allows scientists to calculate the half-life of an isotope undergoing beta decay.
Beta Decay Helps Stabilize Neutron-Rich Nuclei
In neutron-rich nuclei, beta decay plays a crucial role in maintaining stability by converting excess neutrons into protons. This process balances the ratio of neutrons to protons in the nucleus, preventing it from becoming unstable.
Conclusion
In conclusion, the process of beta decay is truly fascinating and has a significant impact on the world of physics. From its discovery to its applications in nuclear medicine, beta decay continues to provide valuable insights into the nature of matter and energy. The ten extraordinary facts about beta decay highlighted in this article demonstrate the complexity and beauty of this fundamental process.By understanding the different types of beta decay, such as beta-minus and beta-plus decay, as well as the role of neutrinos, scientists have been able to explore and investigate the inner workings of atoms and the universe as a whole. Beta decay is not only crucial for explaining the stability of atomic nuclei, but it also plays a vital role in developing technologies like positron emission tomography (PET) scans.As we continue to unravel the mysteries of the universe, beta decay will undoubtedly remain an essential topic in the field of physics. Its implications extend far beyond our everyday lives, shaping our understanding of the fundamental building blocks of matter and the vastness of the cosmos.
FAQs
1. What is beta decay?
Beta decay is a type of radioactive decay in which a nucleus emits either an electron (beta-minus decay) or a positron (beta-plus decay). It is a process that occurs in unstable atomic nuclei.
2. How does beta decay work?
In beta-minus decay, a neutron in the nucleus is converted into a proton, releasing an electron and an antineutrino. In beta-plus decay, a proton transforms into a neutron, emitting a positron and a neutrino.
3. What are the different types of beta decay?
The two main types of beta decay are beta-minus decay and beta-plus decay. Beta-minus decay occurs when a neutron in the nucleus becomes a proton, emitting an electron and an antineutrino. Beta-plus decay involves a proton transforming into a neutron, releasing a positron and a neutrino.
4. Why is beta decay important in nuclear medicine?
Beta decay is crucial in nuclear medicine because it allows the production of radioisotopes that can be used for imaging and treatment. For example, positron emission tomography (PET) scans utilize beta-plus decay to detect and visualize various metabolic processes in the body.
5. How does beta decay contribute to our understanding of the universe?
Studying beta decay provides insights into the nature of matter and the universe. By examining the properties of beta particles and neutrinos, scientists can gain a deeper understanding of the fundamental forces and particles that govern our universe.
Captivated by beta decay's extraordinary facts? Continue your exploration of radioactive phenomena with our article on alpha decay. Unravel the secrets behind this fundamental nuclear process, from its role in radiotherapy to its impact on elemental transmutations. Prepare to be amazed as you delve deeper into the world of radioactive decay and its significance in various scientific fields. Don't miss out on this opportunity to expand your knowledge and gain a newfound appreciation for the complex workings of the atomic nucleus.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
