What is theoretical yield? In chemistry, the theoretical yield is the maximum amount of product that can be produced in a chemical reaction based on the amount of limiting reactant. It's calculated using stoichiometry, which involves balanced chemical equations to determine the proportions of reactants and products. Theoretical yield assumes perfect conditions where no product is lost, and all reactants are converted to products. However, in real-life experiments, the actual yield is often less due to factors like incomplete reactions, side reactions, or loss of product during recovery. Understanding theoretical yield helps chemists predict the efficiency of reactions and optimize conditions for better results.
What is Theoretical Yield?
Theoretical yield is a concept in chemistry that refers to the maximum amount of product that can be generated from a given amount of reactants. This assumes perfect conditions where no losses occur during the reaction.
- Theoretical yield is calculated based on stoichiometry, which involves the balanced chemical equation of the reaction.
- It represents the ideal scenario, meaning it assumes 100% efficiency in the reaction process.
- Theoretical yield is always higher than the actual yield obtained in practical experiments.
- It helps chemists understand the efficiency of a reaction by comparing it to the actual yield.
Importance of Theoretical Yield
Understanding theoretical yield is crucial for several reasons. It helps in planning and optimizing chemical reactions, ensuring resources are used efficiently.
- It allows chemists to predict the amount of product that can be expected from a reaction.
- Theoretical yield helps in budgeting and resource allocation for industrial chemical processes.
- It aids in identifying the limiting reactant, which is the reactant that will be completely consumed first, stopping the reaction.
- By comparing theoretical and actual yields, chemists can identify inefficiencies and potential improvements in the reaction process.
Calculating Theoretical Yield
Calculating theoretical yield involves a few steps, starting with a balanced chemical equation and using molar ratios.
- First, balance the chemical equation to ensure the law of conservation of mass is followed.
- Convert the amounts of reactants from grams to moles using their molar masses.
- Use the stoichiometric coefficients from the balanced equation to determine the mole ratio between reactants and products.
- Calculate the moles of product expected based on the limiting reactant.
- Convert the moles of product back to grams using the molar mass of the product.
Factors Affecting Theoretical Yield
Several factors can influence the theoretical yield, making it an essential consideration in chemical reactions.
- Purity of reactants: Impurities can reduce the amount of product formed.
- Measurement accuracy: Inaccurate measurements of reactants can lead to incorrect yield predictions.
- Reaction conditions: Temperature, pressure, and catalysts can affect the efficiency of a reaction.
- Side reactions: Unintended reactions can consume reactants and produce undesired products, reducing the yield.
Theoretical Yield vs. Actual Yield
Theoretical yield is often compared to actual yield to determine the efficiency of a reaction.
- Actual yield is the amount of product actually obtained from a reaction.
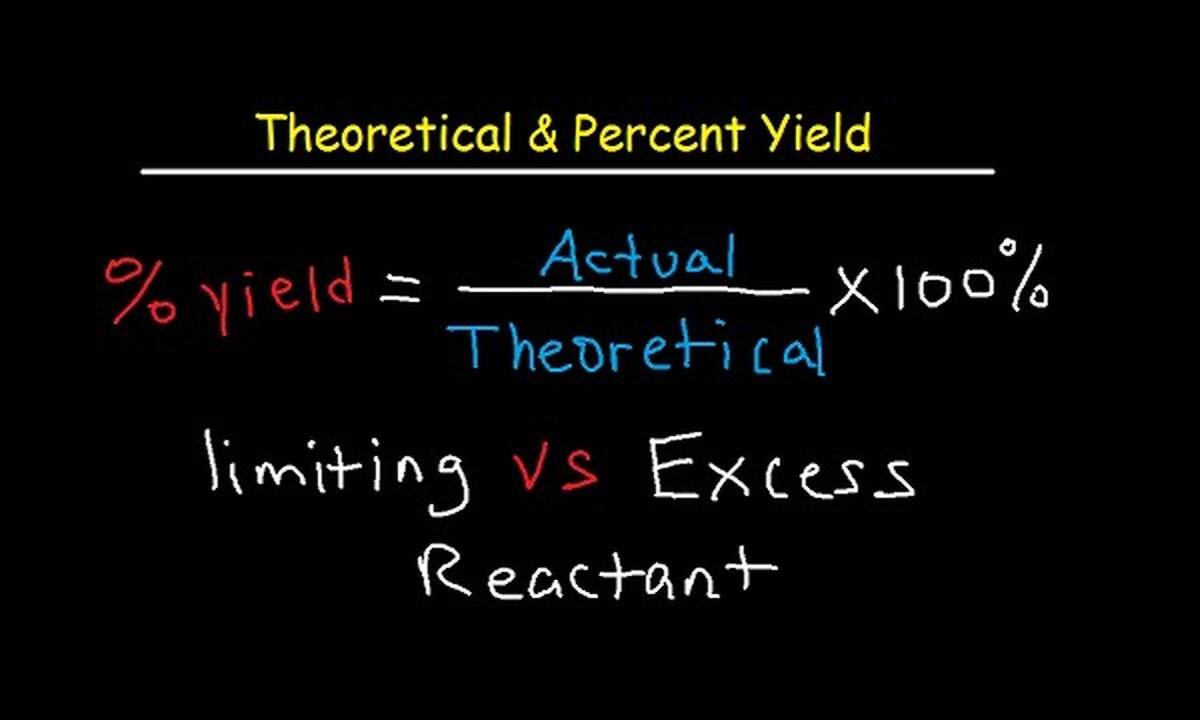
- The percentage yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100.
- A high percentage yield indicates an efficient reaction, while a low percentage yield suggests inefficiencies or losses.
- Factors such as incomplete reactions, side reactions, and product loss during purification can lower the actual yield.
Real-World Applications of Theoretical Yield
Theoretical yield has practical applications in various fields, from pharmaceuticals to environmental science.
- In pharmaceuticals, it helps in scaling up reactions for drug production.
- Environmental scientists use theoretical yield to predict the outcomes of chemical reactions in natural processes.
- Industrial chemists rely on it to optimize manufacturing processes and reduce waste.
- It is used in academic research to design experiments and interpret results.
Challenges in Achieving Theoretical Yield
Achieving the theoretical yield in practice is challenging due to various factors.
- Incomplete reactions: Not all reactants may fully convert to products.
- Side reactions: Competing reactions can consume reactants and produce unwanted products.
- Losses during purification: Some product may be lost during isolation and purification steps.
- Measurement errors: Inaccurate measurements of reactants can lead to discrepancies between theoretical and actual yields.
Improving Yield in Chemical Reactions
Chemists employ various strategies to improve the yield of chemical reactions.
- Using excess reactants: Adding more of the limiting reactant can drive the reaction to completion.
- Optimizing reaction conditions: Adjusting temperature, pressure, and catalysts can enhance reaction efficiency.
- Purifying reactants: Removing impurities can increase the yield of the desired product.
- Minimizing side reactions: Careful control of reaction conditions can reduce the occurrence of side reactions.
Theoretical Yield in Education
The concept of theoretical yield is an essential part of chemistry education.
- It helps students understand the principles of stoichiometry and chemical reactions.
- Laboratory experiments often involve calculating theoretical yield to compare with actual results.
- Understanding theoretical yield prepares students for careers in chemistry and related fields.
- It reinforces the importance of accuracy and precision in scientific measurements.
Fun Facts About Theoretical Yield
Here are some interesting tidbits about theoretical yield that you might find surprising.
- The concept of theoretical yield dates back to the early days of chemistry when scientists first began to understand chemical reactions.
- Theoretical yield calculations can be applied to everyday situations, such as cooking recipes, where ingredients must be measured and combined in specific ratios.
Final Thoughts on Theoretical Yield
Understanding theoretical yield is crucial for anyone diving into chemistry. It helps predict the maximum amount of product possible from a given set of reactants. This concept isn't just academic; it has real-world applications in industries like pharmaceuticals, agriculture, and manufacturing. Knowing how to calculate theoretical yield can save time, resources, and money.
Remember, theoretical yield assumes perfect conditions—something rarely achieved in practice. Factors like impurities, measurement errors, and incomplete reactions often lead to a lower actual yield. However, aiming for the theoretical yield gives a benchmark for efficiency.
So, whether you're a student, a professional chemist, or just curious, grasping this concept can provide valuable insights into chemical reactions. Keep experimenting, stay curious, and let the wonders of chemistry unfold.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
