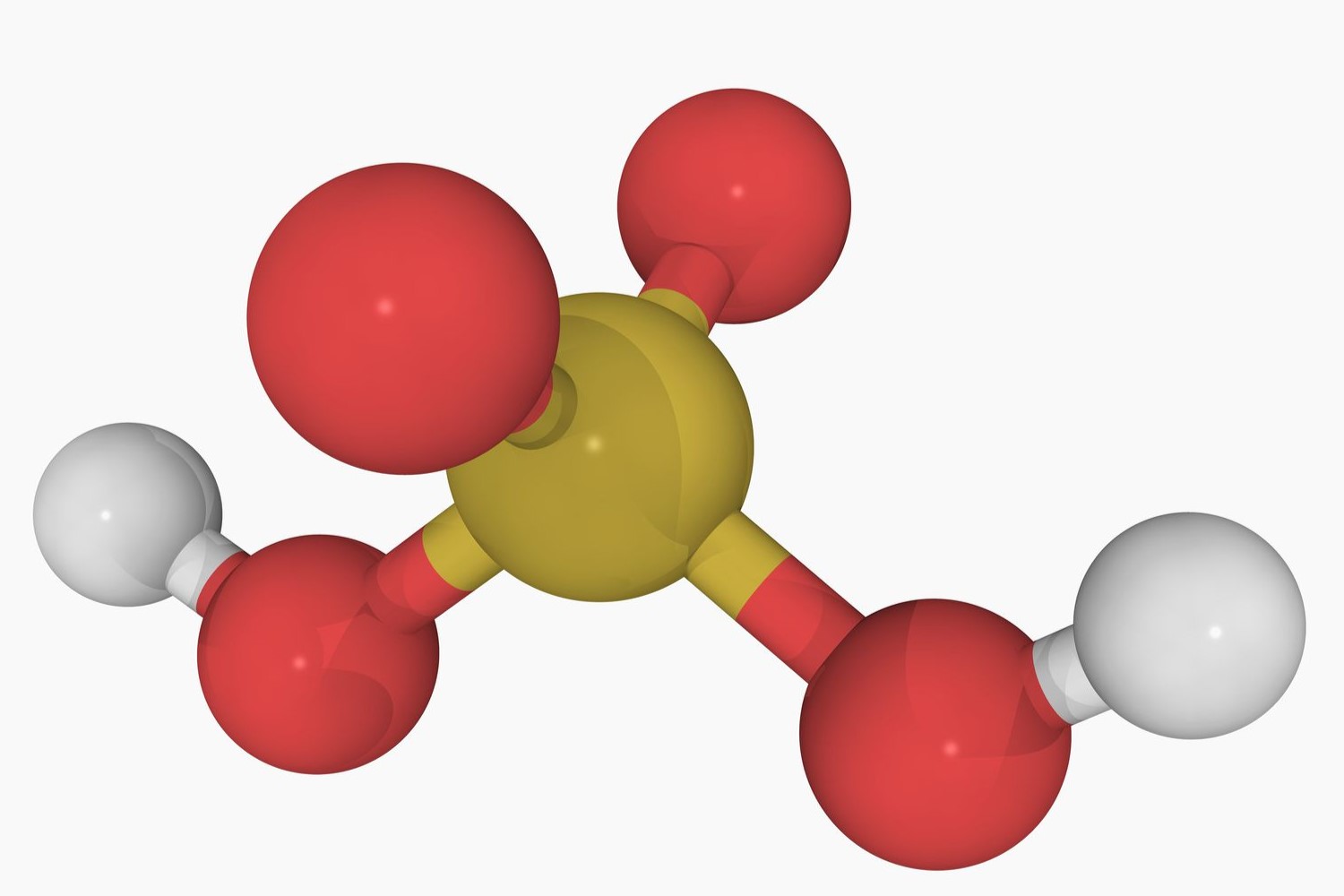
Polyprotic acids are fascinating because they can donate more than one proton per molecule. This unique property makes them essential in various chemical reactions and processes. Understanding these acids can help in fields like biochemistry, environmental science, and industrial chemistry. For instance, sulfuric acid, a common polyprotic acid, plays a crucial role in manufacturing fertilizers, batteries, and cleaning agents. Learning about polyprotic acids can also aid in grasping concepts like pH, buffer solutions, and titration curves. Whether you're a student, a teacher, or just curious, these 36 facts will deepen your appreciation for these versatile compounds.
What is Polyprotic Acid?
Polyprotic acids are fascinating because they can donate more than one proton per molecule. This unique property makes them essential in various chemical reactions and processes. Let's dive into some intriguing facts about these versatile acids.
- Polyprotic acids can donate multiple protons, unlike monoprotic acids that donate just one.
- Common examples include sulfuric acid (H₂SO₄), phosphoric acid (H₃PO₄), and carbonic acid (H₂CO₃).
- Each proton donation occurs in a stepwise manner, with each step having its own dissociation constant.
- The first dissociation constant (Ka1) is usually the highest, making the first proton the easiest to donate.
- Subsequent dissociation constants (Ka2, Ka3, etc.) are progressively lower, indicating more difficulty in donating additional protons.
Importance in Chemistry
Polyprotic acids play a crucial role in various chemical reactions and industrial processes. Their ability to donate multiple protons makes them versatile and valuable.
- Sulfuric acid is widely used in the production of fertilizers, detergents, and batteries.
- Phosphoric acid is a key ingredient in soft drinks, providing a tangy flavor.
- Carbonic acid forms when carbon dioxide dissolves in water, playing a significant role in the carbonation of beverages.
- These acids are also essential in buffer solutions, helping to maintain pH stability in biological and chemical systems.
- Polyprotic acids are used in titration experiments to determine the concentration of unknown solutions.
Biological Significance
Polyprotic acids are not just important in chemistry labs; they also have significant roles in biological systems.
- Phosphoric acid is a component of DNA and RNA, the molecules that carry genetic information.
- Carbonic acid helps regulate blood pH through the bicarbonate buffer system.
- Citric acid, another polyprotic acid, is a key intermediate in the citric acid cycle, a crucial metabolic pathway.
- Amino acids like glutamic acid and aspartic acid are polyprotic and play vital roles in protein structure and function.
- Polyprotic acids are involved in cellular respiration, helping to produce energy in cells.
Environmental Impact
The environmental impact of polyprotic acids is significant, influencing various natural processes and human activities.
- Acid rain, primarily caused by sulfuric and nitric acids, can harm ecosystems and man-made structures.
- Ocean acidification, driven by increased carbon dioxide levels, leads to higher concentrations of carbonic acid, affecting marine life.
- Polyprotic acids are used in wastewater treatment to neutralize alkaline waste and remove heavy metals.
- They play a role in soil chemistry, affecting nutrient availability and plant growth.
- Industrial emissions of polyprotic acids must be carefully managed to prevent environmental damage.
Industrial Applications
Polyprotic acids are indispensable in many industrial applications due to their unique properties.
- Sulfuric acid is used in petroleum refining to remove impurities.
- Phosphoric acid is employed in the production of rust inhibitors and cleaning agents.
- Carbonic acid is used in the beverage industry for carbonation.
- Polyprotic acids are involved in the manufacture of pharmaceuticals, providing the necessary acidity for certain reactions.
- They are used in electroplating to deposit metal coatings on various surfaces.
Challenges and Considerations
Working with polyprotic acids comes with its own set of challenges and considerations.
- Handling these acids requires proper safety measures due to their corrosive nature.
- Accurate measurement of dissociation constants is crucial for understanding their behavior in solutions.
- Polyprotic acids can form complex ions with metals, complicating chemical reactions.
- Their multiple dissociation steps can make pH calculations more complex.
- Storage and disposal of polyprotic acids must be managed carefully to prevent environmental contamination.
Fun Facts
Let's end with some fun and lesser-known facts about polyprotic acids.
- The fizz in soft drinks is due to carbonic acid, a polyprotic acid formed when CO₂ dissolves in water.
- Sulfuric acid is often called the "king of chemicals" due to its wide range of applications.
- Phosphoric acid is used in dentistry to etch teeth before applying fillings or sealants.
- Citric acid, found in citrus fruits, is a natural preservative and flavor enhancer.
- Some polyprotic acids can act as both acids and bases, depending on the pH of the solution.
- The study of polyprotic acids dates back to ancient alchemy, where early chemists explored their properties and uses.
Final Thoughts on Polyprotic Acids
Polyprotic acids, with their ability to donate more than one proton, play a crucial role in chemistry. They exhibit unique behaviors in titration curves, making them fascinating subjects for study. Understanding their dissociation constants helps predict their reactions in various environments. These acids are found in everyday substances like phosphoric acid in sodas and sulfuric acid in car batteries. Their applications span from industrial processes to biological systems, highlighting their versatility. By grasping the basics of polyprotic acids, you can better appreciate their significance in both scientific and practical contexts. Whether you're a student, a professional, or just curious, knowing these facts enriches your knowledge of the chemical world. Keep exploring, and you'll uncover even more intriguing aspects of these multifaceted compounds.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.