Lattice energy might sound like a complex term, but it’s a fundamental concept in chemistry. What is lattice energy? Lattice energy is the energy released when ions bond to form a crystalline lattice. This energy is crucial because it influences the stability and properties of ionic compounds. Understanding lattice energy helps explain why certain salts dissolve in water while others don't. It also sheds light on why some materials have high melting points. In this post, we'll explore 36 fascinating facts about lattice energy that will deepen your understanding of this essential chemical concept. Get ready to dive into the world of ionic bonds and crystal lattices!
What is Lattice Energy?
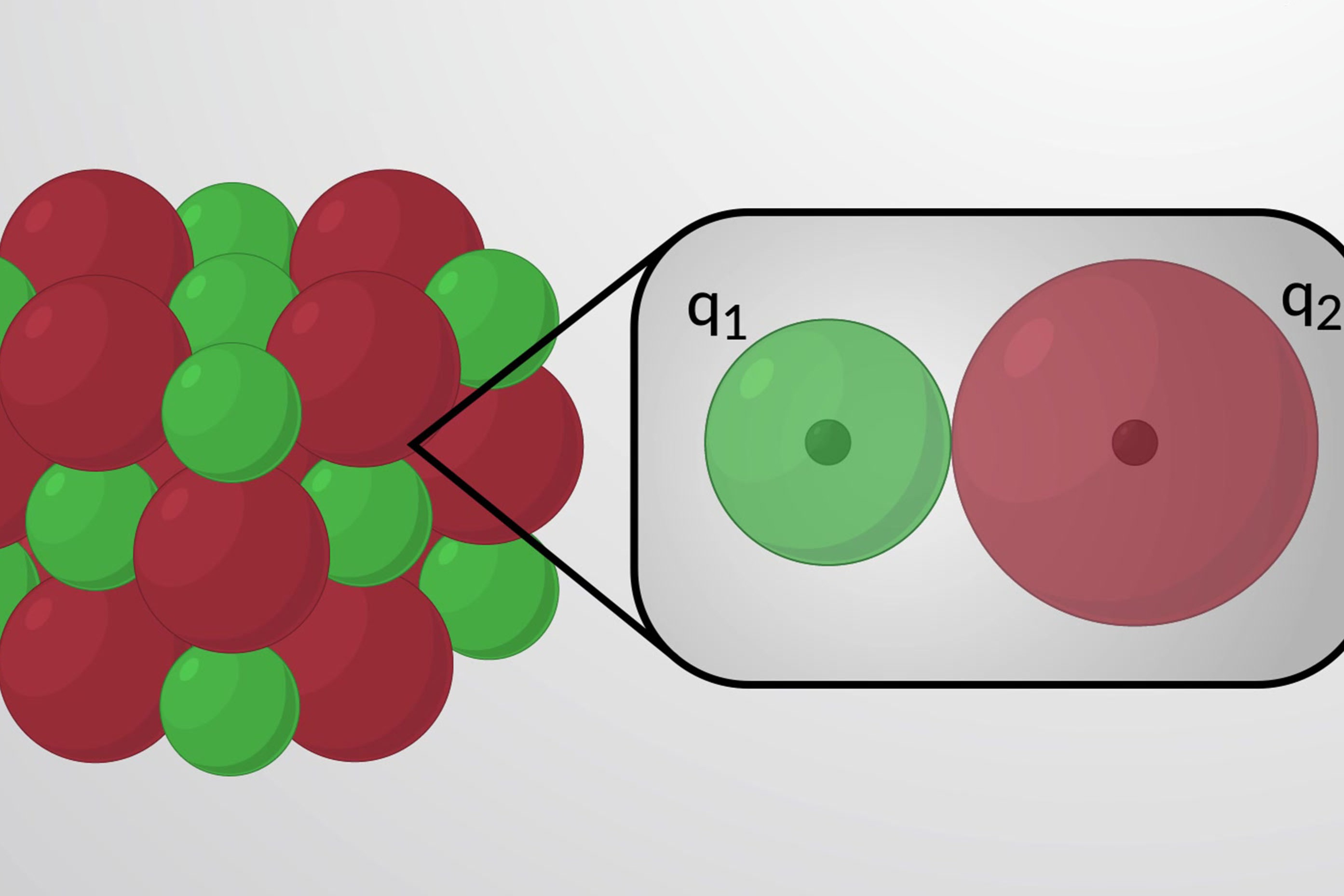
Lattice energy is a measure of the strength of the bonds in an ionic compound. It represents the energy required to separate one mole of a solid ionic compound into its gaseous ions. This concept is crucial in understanding the stability and properties of ionic compounds.
-
Lattice energy is always positive because it requires energy to break the ionic bonds in a solid compound.
-
Measured in kilojoules per mole (kJ/mol), lattice energy quantifies the energy needed to separate ions in a crystal lattice.
-
Born-Haber cycle is a method used to calculate lattice energy indirectly by considering the formation of an ionic compound from its elements.
Factors Affecting Lattice Energy
Several factors influence the magnitude of lattice energy. Understanding these factors helps in predicting the properties of ionic compounds.
-
Ionic charge: Higher charges on the ions result in greater lattice energy due to stronger electrostatic forces.
-
Ionic radius: Smaller ions lead to higher lattice energy because they can get closer together, increasing the electrostatic attraction.
-
Crystal structure: The arrangement of ions in the crystal lattice affects the lattice energy. Different structures can lead to variations in bond strength.
Importance of Lattice Energy
Lattice energy plays a significant role in various chemical properties and reactions. It helps in understanding the behavior of ionic compounds.
-
Solubility: Compounds with high lattice energy are generally less soluble in water because more energy is required to break the ionic bonds.
-
Melting and boiling points: Higher lattice energy usually means higher melting and boiling points due to the strong ionic bonds that need more energy to break.
-
Stability: Ionic compounds with high lattice energy are more stable because of the strong attraction between ions.
Calculating Lattice Energy
There are different methods to calculate lattice energy, each with its own set of principles and applications.
-
Born-Haber cycle: This method involves a series of steps, including ionization energy, electron affinity, and enthalpy of formation, to calculate lattice energy.
-
Kapustinskii equation: An empirical formula that estimates lattice energy based on ionic charges and radii.
-
Coulomb's law: This law can be used to calculate the electrostatic energy between ions, which contributes to the lattice energy.
Real-World Applications
Lattice energy has practical applications in various fields, from material science to pharmaceuticals.
-
Battery technology: Understanding lattice energy helps in designing better electrolytes and electrodes for batteries.
-
Pharmaceuticals: Lattice energy influences the solubility and stability of ionic drugs, affecting their efficacy.
-
Material science: It helps in designing materials with desired properties, such as high melting points or specific solubility.
Interesting Facts About Lattice Energy
Here are some intriguing facts that highlight the importance and complexity of lattice energy.
-
Magnesium oxide (MgO) has one of the highest lattice energies among ionic compounds, making it extremely stable.
-
Lattice energy can be endothermic: While usually exothermic, some processes involving lattice energy can absorb heat.
-
Predicting properties: Lattice energy can be used to predict the hardness and brittleness of ionic compounds.
-
Environmental impact: Understanding lattice energy helps in developing environmentally friendly materials by predicting their stability and reactivity.
-
Historical significance: The concept of lattice energy has been crucial in the development of solid-state chemistry and materials science.
Advanced Concepts
For those interested in diving deeper, here are some advanced concepts related to lattice energy.
-
Polarizability: The ability of ions to distort their electron cloud affects lattice energy, with more polarizable ions leading to lower lattice energy.
-
Lattice enthalpy vs. lattice energy: Lattice enthalpy includes the effect of temperature, while lattice energy is measured at absolute zero.
-
Born-Landé equation: A theoretical approach to calculate lattice energy based on electrostatic and repulsive forces.
-
Madelung constant: A factor that accounts for the geometric arrangement of ions in the crystal lattice, affecting the lattice energy.
-
Thermodynamic cycles: These cycles, like the Born-Haber cycle, help in understanding the energy changes during the formation of ionic compounds.
Fun Facts
Lattice energy isn't just a dry scientific concept; it has some fun and surprising aspects too.
-
Table salt (NaCl): Despite being common, it has a relatively high lattice energy, contributing to its stability.
-
Gemstones: The brilliant colors and hardness of gemstones like sapphire and ruby are partly due to their high lattice energies.
-
Explosives: Some explosives rely on the rapid release of lattice energy to produce powerful reactions.
-
Space exploration: Materials with high lattice energy are used in spacecraft to withstand extreme conditions.
-
Everyday life: From the salt in your kitchen to the batteries in your gadgets, lattice energy plays a role in many everyday items.
Lattice Energy in Education
Understanding lattice energy is essential for students and educators in the field of chemistry.
-
Curriculum: Lattice energy is a key topic in high school and college chemistry courses.
-
Experiments: Simple experiments can demonstrate the effects of lattice energy, making it easier to grasp.
-
Textbooks: Many chemistry textbooks include detailed sections on lattice energy, highlighting its importance.
-
Online resources: Numerous websites and videos explain lattice energy, making it accessible to a broader audience.
-
Interactive tools: Software and apps allow students to visualize and calculate lattice energy, enhancing their learning experience.
-
Competitions: Chemistry competitions often include questions on lattice energy, challenging students to apply their knowledge.
Final Thoughts on Lattice Energy
Lattice energy is a fascinating concept in chemistry. It plays a crucial role in determining the stability of ionic compounds. The higher the lattice energy, the more stable the compound. Factors like ionic size and charge significantly impact this energy. Smaller ions with higher charges usually result in higher lattice energy. Understanding this concept helps in predicting the properties of various compounds, like their melting points and solubility.
In practical applications, lattice energy is essential for industries that deal with materials science and pharmaceuticals. It helps chemists design better compounds with desired properties. So, next time you come across an ionic compound, remember the invisible force holding it together is lattice energy. This knowledge not only deepens your understanding of chemistry but also opens doors to various scientific and industrial advancements.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
