The common ion effect is a fascinating concept in chemistry that plays a crucial role in various reactions and solutions. But what exactly is it? Simply put, it refers to the shift in equilibrium that occurs when an ion common to two solutes is added to a solution. This effect can influence solubility, pH levels, and even the formation of precipitates. Understanding this phenomenon is essential for anyone studying chemistry, as it helps explain why certain reactions behave the way they do. Whether you're a student, a teacher, or just curious about science, these 33 facts will shed light on the intriguing world of the common ion effect.
What is the Common Ion Effect?
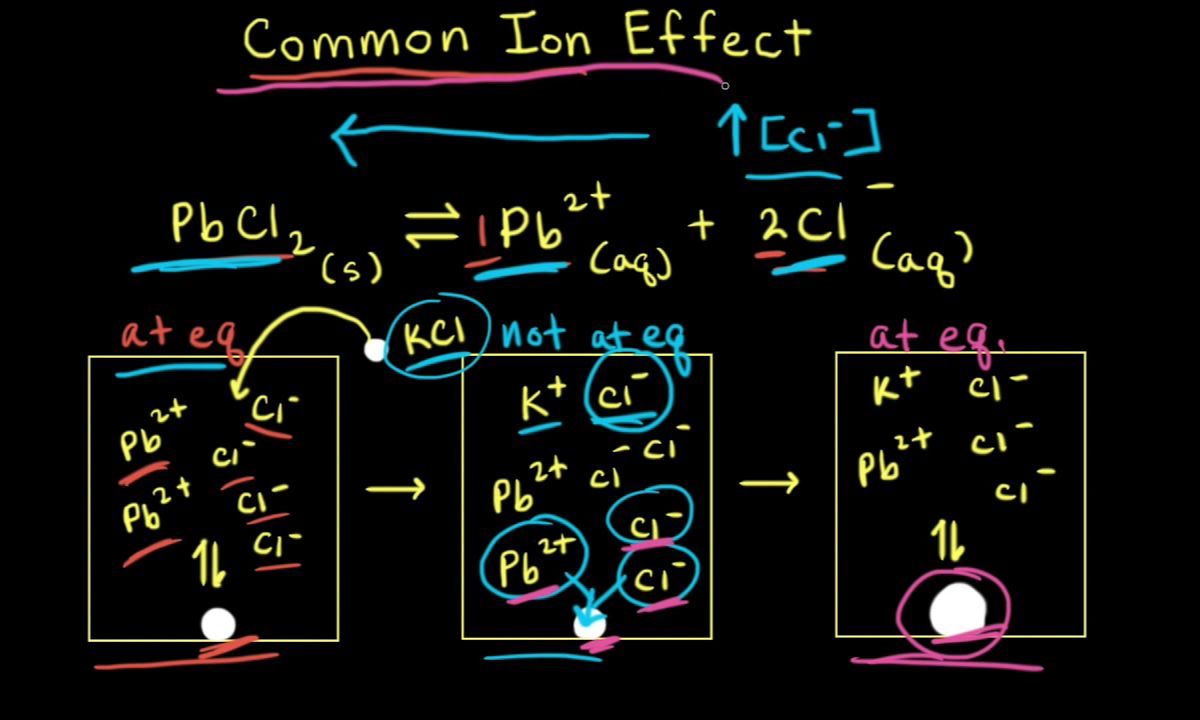
The common ion effect is a phenomenon in chemistry where the addition of an ion common to two solutes brings about precipitation or reduces ionization. This effect is crucial in various chemical processes, including buffer solutions, solubility, and acid-base equilibria.
- The common ion effect occurs when a salt containing an ion already present in the solution is added, reducing the solubility of the salt.
- This effect is a direct application of Le Chatelier's principle, which states that a system in equilibrium will adjust to counteract any changes imposed on it.
- It is often used in analytical chemistry to control the concentration of ions in a solution.
- The common ion effect can be observed in both acidic and basic solutions.
- It plays a significant role in the formation of buffer solutions, which resist changes in pH upon the addition of small amounts of acid or base.
How Does the Common Ion Effect Influence Solubility?
The common ion effect significantly impacts the solubility of compounds. When a common ion is added to a solution, the solubility of a sparingly soluble salt decreases.
- Adding a common ion to a solution containing a sparingly soluble salt decreases the salt's solubility.
- This decrease in solubility occurs because the added common ion shifts the equilibrium towards the formation of the undissolved salt.
- For example, adding sodium chloride to a solution of silver chloride decreases the solubility of silver chloride.
- The solubility product constant (Ksp) helps predict the extent of the common ion effect on solubility.
- In industrial processes, the common ion effect is used to precipitate unwanted ions from solutions.
Applications in Buffer Solutions
Buffer solutions are essential in maintaining a stable pH in various chemical and biological systems. The common ion effect is vital in the preparation and function of these buffers.
- Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base.
- The common ion effect helps maintain the pH of buffer solutions by shifting the equilibrium of weak acids or bases.
- For instance, adding sodium acetate to an acetic acid solution creates a buffer that resists changes in pH.
- Biological systems, such as blood, rely on buffer solutions to maintain a stable pH for proper functioning.
- The common ion effect is also used in pharmaceutical formulations to stabilize the pH of drug solutions.
Role in Acid-Base Equilibria
The common ion effect plays a crucial role in acid-base equilibria, influencing the ionization of weak acids and bases.
- Adding a common ion to a weak acid or base solution suppresses its ionization.
- This suppression occurs because the added ion shifts the equilibrium towards the non-ionized form of the acid or base.
- For example, adding sodium acetate to acetic acid suppresses the ionization of acetic acid.
- The common ion effect is used in titration to control the pH of the solution.
- It is also essential in the preparation of standard solutions in analytical chemistry.
Industrial and Environmental Applications
The common ion effect has various industrial and environmental applications, from water treatment to metal extraction.
- In water treatment, the common ion effect is used to precipitate unwanted ions, making the water safe for consumption.
- It is also used in the extraction of metals from ores by precipitating metal ions from solution.
- The common ion effect helps control the pH of industrial processes, ensuring optimal conditions for chemical reactions.
- In environmental science, it is used to remove heavy metals from wastewater.
- The common ion effect is also employed in the production of high-purity chemicals by precipitating impurities.
Common Ion Effect in Everyday Life
The common ion effect is not just limited to laboratories and industries; it also plays a role in everyday life.
- Hard water contains high concentrations of calcium and magnesium ions, which can be precipitated using the common ion effect.
- The common ion effect is used in cooking, such as when adding salt to boiling water to cook pasta.
- It is also observed in the preservation of food, where salt is used to precipitate unwanted ions and prevent spoilage.
- In medicine, the common ion effect is used to control the solubility of drugs in the body.
- The common ion effect is also used in aquariums to maintain the water quality for fish and other aquatic organisms.
Interesting Facts About the Common Ion Effect
Here are some intriguing facts about the common ion effect that highlight its importance and versatility.
- The common ion effect can be used to separate ions in a mixture by selectively precipitating one ion while leaving others in solution.
- It is a key concept in the study of chemical equilibria and is often taught in introductory chemistry courses.
- The common ion effect can be used to control the rate of chemical reactions by influencing the concentration of reactants and products.
Final Thoughts on the Common Ion Effect
Understanding the common ion effect can be a game-changer in chemistry. It helps predict how adding a common ion to a solution affects solubility and pH levels. This principle is crucial in various fields, from pharmaceuticals to environmental science. Knowing how to manipulate ion concentrations can lead to more effective drug formulations and better water treatment processes.
Grasping this concept also aids in mastering buffer solutions, which are essential in maintaining stable pH levels in biological systems. Whether you're a student, a professional, or just curious, the common ion effect is a fundamental topic that offers practical applications and deeper insights into chemical behavior.
Keep exploring and experimenting. The more you understand these principles, the more you'll appreciate the intricate dance of ions in every solution. Happy learning!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
