What is the atomic radius? The atomic radius is the distance from an atom's nucleus to the outer boundary of its electron cloud. This measurement helps scientists understand the size of atoms, which varies across the periodic table. Elements on the left side of the table generally have larger atomic radii, while those on the right have smaller ones. Factors like the number of electron shells and the effective nuclear charge influence these variations. Knowing the atomic radius is crucial for predicting how atoms will interact in chemical reactions. Dive into these 33 fascinating facts about atomic radius to learn more!
What is Atomic Radius?
Understanding atomic radius is crucial in chemistry. It refers to the size of an atom, typically measured from the nucleus to the outer boundary of the surrounding cloud of electrons.
- Atomic radius is usually measured in picometers (pm) or angstroms (Å). One angstrom equals 100 picometers.
- The atomic radius can vary depending on the atom's state, such as whether it is isolated or part of a molecule.
How is Atomic Radius Measured?
Measuring atomic radius isn't straightforward. Different methods yield slightly different results.
- X-ray crystallography is a common method used to measure atomic radius. It involves analyzing the diffraction patterns of X-rays passing through a crystal.
- Another method is electron microscopy, which uses electron beams to visualize atoms and measure their sizes.
Factors Affecting Atomic Radius
Several factors influence the size of an atom. These include the number of protons, electrons, and the electron configuration.
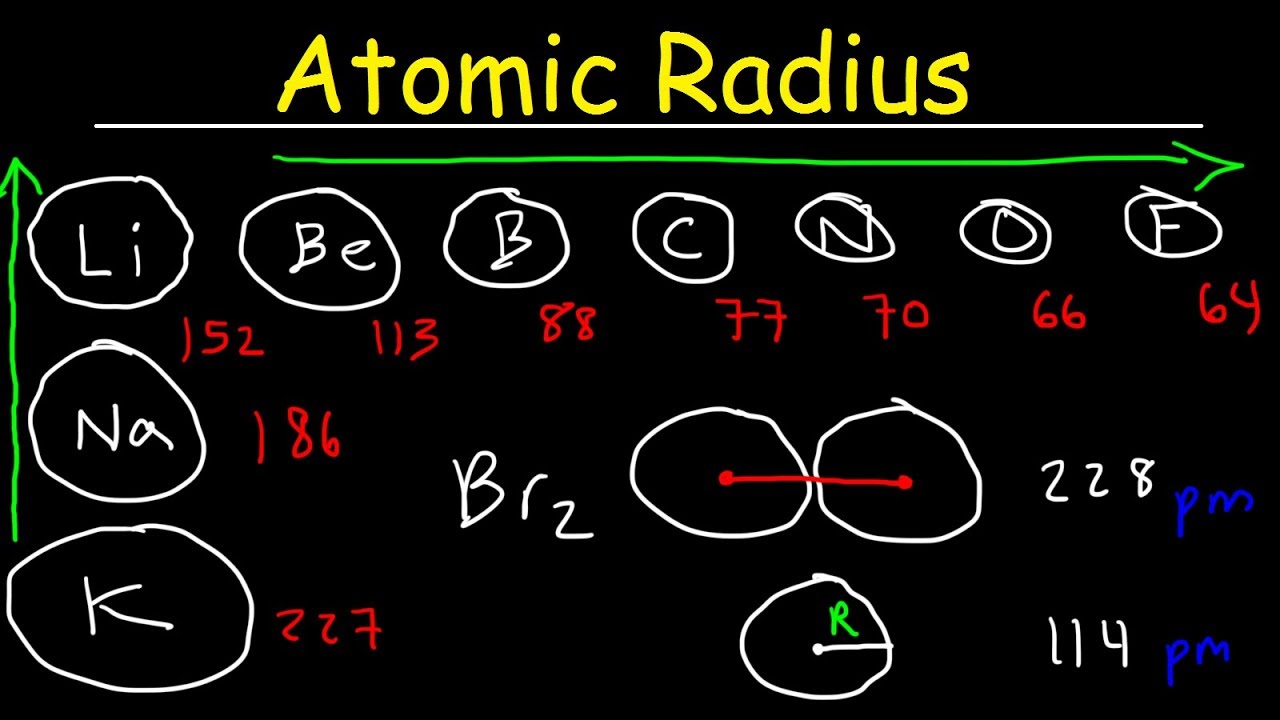
- Atomic radius generally decreases across a period from left to right on the periodic table. This happens because the number of protons increases, pulling electrons closer to the nucleus.
- Atomic radius increases down a group in the periodic table. Additional electron shells are added, making the atom larger.
- The effective nuclear charge, which is the net positive charge experienced by electrons, also affects atomic radius. Higher effective nuclear charge pulls electrons closer, reducing the radius.
Trends in the Periodic Table
The periodic table showcases clear trends in atomic radius, helping predict the size of atoms in different elements.
- Alkali metals have the largest atomic radii in their respective periods. They have only one electron in their outer shell, which is far from the nucleus.
- Noble gases have relatively small atomic radii. Their outer electron shells are full, creating a strong pull towards the nucleus.
- Transition metals show less variation in atomic radius across a period. Their inner d-electrons shield the outer electrons from the nucleus's pull.
Comparing Atomic Radius with Ionic Radius
Atomic radius and ionic radius are related but distinct concepts. Ionic radius refers to the size of an ion, which can differ significantly from the neutral atom.
- Cations, or positively charged ions, have smaller radii than their neutral atoms. Losing electrons reduces electron-electron repulsion, allowing the remaining electrons to be pulled closer to the nucleus.
- Anions, or negatively charged ions, have larger radii than their neutral atoms. Gaining electrons increases electron-electron repulsion, pushing electrons further apart.
Applications of Atomic Radius
Understanding atomic radius has practical applications in various scientific fields.
- In materials science, atomic radius helps predict how atoms will pack together in a solid. This affects the material's properties, such as density and hardness.
- In chemistry, atomic radius influences reactivity. Smaller atoms with higher effective nuclear charge tend to be more reactive.
- Atomic radius is crucial in nanotechnology. Manipulating atoms at the nanoscale requires precise knowledge of their sizes.
Interesting Facts About Atomic Radius
Here are some intriguing tidbits about atomic radius that highlight its complexity and importance.
- Helium has the smallest atomic radius of all elements. Its electrons are very close to the nucleus due to the high effective nuclear charge.
- Francium has one of the largest atomic radii. It is highly reactive and has a very low ionization energy.
- Atomic radius can be affected by the atom's environment. For example, atoms in a solid lattice may have different radii than isolated atoms.
- The concept of atomic radius was first introduced by German chemist Wolfgang Pauli in the early 20th century.
- Atomic radius is not a fixed value. It can change depending on the atom's energy state and bonding situation.
- The van der Waals radius is another way to measure atomic size. It considers the distance between non-bonded atoms in a molecule.
- Atomic radius plays a role in determining the boiling and melting points of elements. Larger atoms generally have lower boiling and melting points.
- In metallic bonding, atoms are packed closely together, resulting in smaller atomic radii compared to their isolated states.
- Atomic radius can influence the color of compounds. For example, smaller atoms can cause shifts in the absorption of light, changing the compound's color.
- The atomic radius of hydrogen is about 53 picometers, making it one of the smallest atoms.
- Atomic radius can be used to predict the strength of intermolecular forces. Smaller atoms with higher effective nuclear charge tend to form stronger intermolecular bonds.
- The concept of atomic radius is essential in quantum chemistry. It helps in understanding the behavior of electrons in atoms and molecules.
- Atomic radius can affect the solubility of compounds. Larger atoms may create more space within a crystal lattice, affecting how easily the compound dissolves.
- In biology, atomic radius is important for understanding how atoms interact within biomolecules like proteins and DNA.
- Atomic radius can influence the electrical conductivity of materials. Smaller atoms with tightly bound electrons tend to be better conductors.
- The atomic radius of an element can be used to predict its place in the periodic table, even if the element has not been discovered yet.
- Atomic radius is a key factor in determining the acidity of elements. Smaller atoms with higher effective nuclear charge tend to form stronger acids.
- The study of atomic radius continues to evolve with advancements in technology, providing deeper insights into the nature of atoms and their interactions.
Final Thoughts on Atomic Radius
Atomic radius is a fascinating concept that helps us understand the size of atoms and their behavior in different environments. It varies across the periodic table, influenced by factors like electron shells and nuclear charge. Elements in the same group have similar atomic radii, while those in the same period show a trend of decreasing radius from left to right.
Understanding atomic radius is crucial for grasping chemical bonding, reactivity, and properties of elements. It plays a key role in predicting how atoms will interact in molecules and compounds. This knowledge is fundamental for fields like chemistry, physics, and materials science.
By grasping these facts, you gain a deeper appreciation for the microscopic world and the forces shaping the universe. Keep exploring and questioning, as there's always more to learn about the building blocks of matter.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
