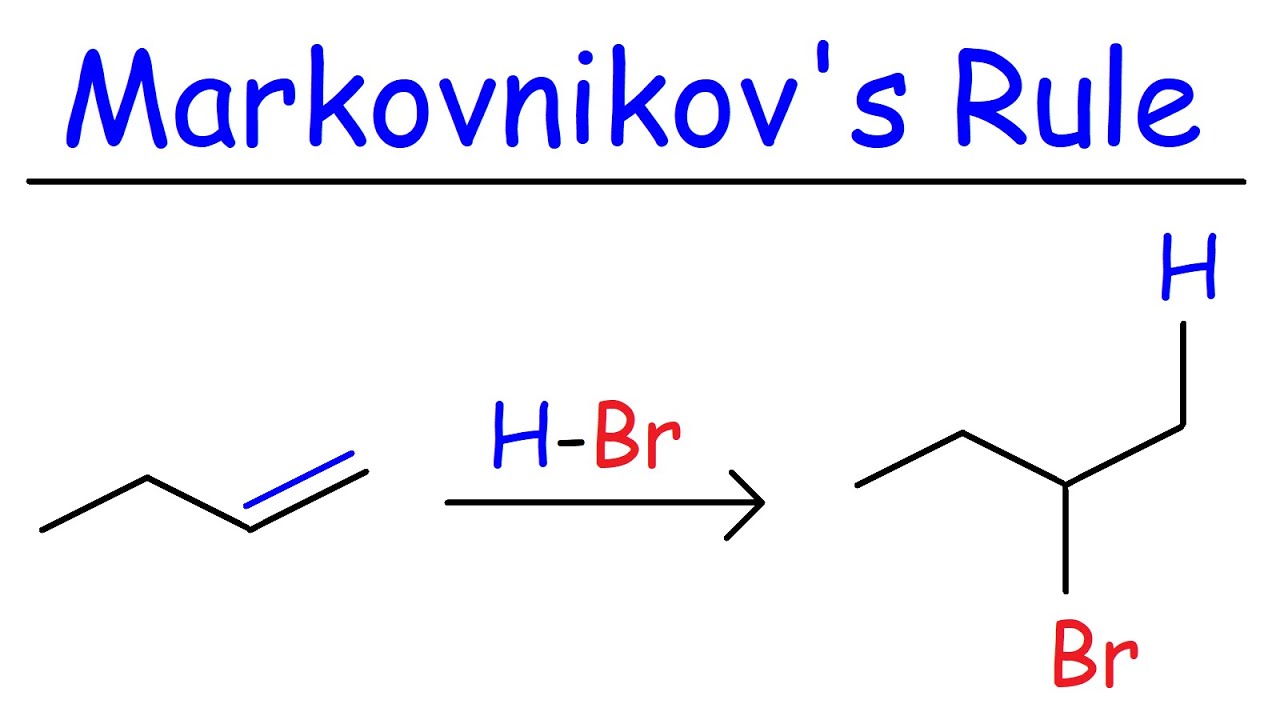
Markovnikov's rule is a fundamental concept in organic chemistry that helps predict the outcome of addition reactions involving alkenes. But what exactly is Markovnikov's rule? In simple terms, it states that when a protic acid (like HCl or HBr) adds to an asymmetric alkene, the hydrogen atom attaches to the carbon with more hydrogen atoms, while the halide (or other substituent) attaches to the carbon with fewer hydrogen atoms. This rule guides chemists in understanding how molecules behave during reactions, making it easier to predict products. Whether you're a student, a chemistry enthusiast, or just curious, these 32 facts will deepen your understanding of this essential rule.
What is Markovnikov's Rule?
Markovnikov's rule is a principle in organic chemistry that helps predict the outcome of certain chemical reactions. It was formulated by the Russian chemist Vladimir Markovnikov in 1869. This rule is particularly useful in understanding how molecules add to alkenes and alkynes.
-
Markovnikov's rule states that during the addition of a protic acid (HX) to an alkene, the hydrogen atom (H) attaches to the carbon with the greater number of hydrogen atoms already attached, while the halide (X) attaches to the carbon with fewer hydrogen atoms.
-
This rule helps chemists predict the major product of an addition reaction, making it easier to synthesize specific compounds.
Historical Background
Understanding the history behind Markovnikov's rule can provide context for its significance in chemistry.
-
Vladimir Markovnikov was born in 1838 in Russia and made significant contributions to organic chemistry during his career.
-
He formulated his famous rule while studying the addition reactions of hydrogen halides to alkenes.
-
Markovnikov's work was initially met with skepticism but gained acceptance as more experimental evidence supported his findings.
Applications in Organic Chemistry
Markovnikov's rule has numerous applications in organic chemistry, particularly in the synthesis of complex molecules.
-
It is commonly used in the petrochemical industry to predict the products of hydrocarbon reactions.
-
The rule is also essential in the pharmaceutical industry for designing drugs with specific molecular structures.
-
Markovnikov's rule helps chemists understand the behavior of alkenes and alkynes in various chemical reactions.
Exceptions to the Rule
While Markovnikov's rule is widely applicable, there are notable exceptions that chemists must consider.
-
Anti-Markovnikov addition occurs when the hydrogen atom attaches to the carbon with fewer hydrogen atoms, often facilitated by peroxides.
-
Free radical mechanisms can lead to anti-Markovnikov products, especially in the presence of certain catalysts.
-
Some specific reagents, such as borane (BH3), also lead to anti-Markovnikov addition through hydroboration-oxidation reactions.
Real-World Examples
Examining real-world examples can illustrate the practical importance of Markovnikov's rule.
-
In the production of isopropyl alcohol, propene reacts with water in the presence of an acid catalyst, following Markovnikov's rule.
-
The synthesis of tert-butyl chloride from isobutene and hydrochloric acid is another example where Markovnikov's rule predicts the major product.
-
The formation of 2-bromopropane from propene and hydrobromic acid showcases the rule's predictive power.
Importance in Academic Research
Markovnikov's rule continues to be a topic of interest in academic research and education.
-
It is a fundamental concept taught in undergraduate organic chemistry courses.
-
Researchers study the rule to develop new catalysts and reaction conditions that can control product distribution.
-
Markovnikov's rule is often cited in scientific literature when discussing the regioselectivity of addition reactions.
Advanced Concepts
For those delving deeper into organic chemistry, understanding advanced concepts related to Markovnikov's rule is crucial.
-
The rule is based on the stability of carbocation intermediates formed during the reaction.
-
Hyperconjugation and inductive effects play a significant role in determining the stability of these intermediates.
-
Markovnikov's rule can be extended to predict the outcomes of addition reactions involving other electrophiles, not just hydrogen halides.
Fun Facts
Chemistry can be fun, and there are some interesting tidbits about Markovnikov's rule worth knowing.
-
Markovnikov's original paper was published in Russian, and it took years for the scientific community to fully appreciate his work.
-
The rule is sometimes humorously referred to as "Markovnikov's law" in academic circles.
-
Vladimir Markovnikov also made contributions to the field of structural theory in organic chemistry.
Modern Developments
Chemistry is an evolving field, and Markovnikov's rule has seen modern developments and applications.
-
Computational chemistry has provided new insights into the mechanisms behind Markovnikov and anti-Markovnikov additions.
-
Green chemistry initiatives aim to develop more sustainable methods for reactions that follow Markovnikov's rule.
-
Advances in spectroscopy have allowed chemists to study the intermediates in these reactions in greater detail.
Markovnikov's Rule in Popular Culture
Believe it or not, Markovnikov's rule has even made its way into popular culture.
-
The rule has been referenced in TV shows and movies that feature chemistry prominently.
-
It is sometimes used as a plot device in science fiction stories involving chemical reactions.
-
Markovnikov's rule has inspired educational videos and animations that make learning chemistry more engaging.
Future Prospects
Looking ahead, Markovnikov's rule will continue to be a cornerstone of organic chemistry.
-
Ongoing research aims to develop new reactions that can be predicted using Markovnikov's rule.
-
The rule will likely remain a key topic in chemistry education for years to come.
-
As our understanding of chemical reactions deepens, Markovnikov's rule may evolve to encompass new principles and applications.
The Final Word on Markovnikov's Rule
Markovnikov's rule is a cornerstone in organic chemistry. It helps predict how molecules will behave during reactions, especially in the addition of hydrogen halides to alkenes. Named after Vladimir Markovnikov, this rule has been crucial for chemists since the 19th century. It states that the hydrogen atom will attach to the carbon with more hydrogen atoms already present, while the halide will attach to the carbon with fewer hydrogen atoms. This simple yet powerful principle has wide-ranging applications, from creating pharmaceuticals to developing new materials. Understanding Markovnikov's rule can give you a deeper appreciation for the intricate dance of atoms and molecules. So next time you encounter a chemical reaction, remember this rule—it might just help you predict the outcome. Keep exploring, keep questioning, and let your curiosity guide you through the fascinating world of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
