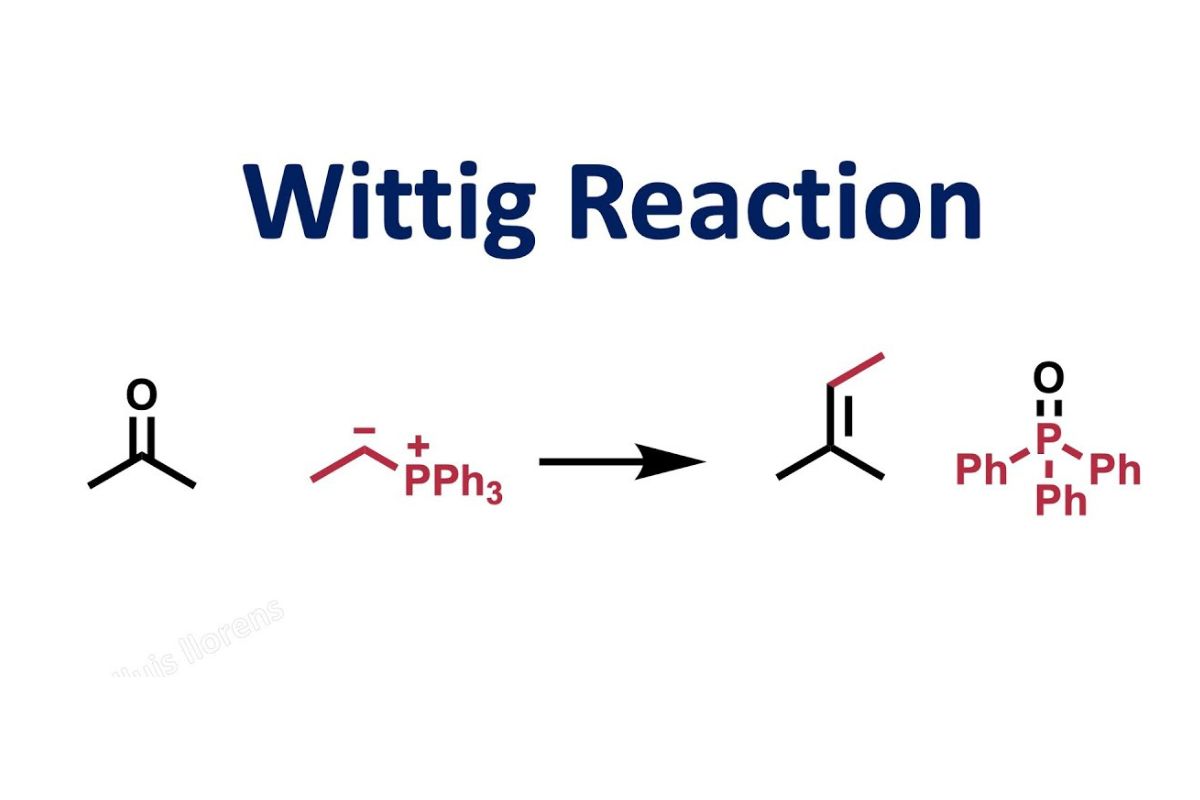
What is the Wittig reaction? The Wittig reaction is a chemical reaction used to convert aldehydes or ketones into alkenes. Named after German chemist Georg Wittig, who won the Nobel Prize in Chemistry in 1979 for this discovery, it involves the reaction of a phosphonium ylide with a carbonyl compound. This reaction is highly valued in organic chemistry for its ability to form carbon-carbon double bonds with precision. It's a go-to method for synthesizing complex molecules, especially in pharmaceuticals and materials science. Whether you're a student, a researcher, or just curious, understanding the Wittig reaction can open doors to mastering organic synthesis.
What is the Wittig Reaction?
The Wittig reaction is a chemical reaction used to convert aldehydes or ketones into alkenes. This reaction is named after the German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for this discovery.
- The Wittig reaction was discovered by Georg Wittig in 1954.
- It involves the reaction of a phosphonium ylide with an aldehyde or ketone.
- The reaction produces an alkene and a triphenylphosphine oxide byproduct.
- Wittig reactions are widely used in organic synthesis to form carbon-carbon double bonds.
- The reaction is highly selective, usually producing the Z-alkene as the major product.
How Does the Wittig Reaction Work?
Understanding the mechanism of the Wittig reaction helps in grasping its significance in organic chemistry. The process involves several steps that lead to the formation of the desired product.
- The reaction starts with the formation of a phosphonium ylide from a phosphonium salt.
- The ylide then reacts with the carbonyl compound (aldehyde or ketone).
- A four-membered ring intermediate called an oxaphosphetane is formed.
- The oxaphosphetane decomposes to yield the alkene and triphenylphosphine oxide.
- The reaction mechanism is concerted, meaning all bond-making and bond-breaking steps occur simultaneously.
Applications of the Wittig Reaction
The Wittig reaction has numerous applications in the field of organic chemistry, particularly in the synthesis of complex molecules.
- It is used in the synthesis of natural products, such as vitamins and hormones.
- The reaction is employed in the pharmaceutical industry to create drug molecules.
- It is also used in the production of fragrances and flavors.
- Wittig reactions are crucial in the development of polymers and materials science.
- The reaction is used in academic research to study reaction mechanisms and develop new synthetic methods.
Advantages of the Wittig Reaction
The Wittig reaction offers several advantages that make it a valuable tool in organic synthesis.
- It provides a straightforward method to form carbon-carbon double bonds.
- The reaction is highly selective, often producing a single isomer.
- It can be performed under mild conditions, making it suitable for sensitive substrates.
- The reaction is versatile, allowing for the synthesis of a wide range of alkenes.
- It is compatible with various functional groups, enabling complex molecule synthesis.
Limitations of the Wittig Reaction
Despite its many advantages, the Wittig reaction has some limitations that need to be considered.
- The reaction can produce a mixture of E- and Z-alkenes, complicating product isolation.
- It requires the use of phosphonium ylides, which can be challenging to prepare.
- The reaction can be sensitive to steric hindrance, affecting yields.
- It may not be suitable for substrates with highly reactive functional groups.
- The byproduct, triphenylphosphine oxide, can be difficult to remove from the reaction mixture.
Variations of the Wittig Reaction
Several variations of the Wittig reaction have been developed to address its limitations and expand its scope.
- The Horner-Wadsworth-Emmons reaction uses phosphonate esters instead of phosphonium ylides.
- The Schlosser modification improves the selectivity for E-alkenes.
- The Still-Gennari modification enhances the selectivity for Z-alkenes.
- The Julia-Lythgoe olefination uses sulfonium ylides for alkene synthesis.
- The Peterson olefination employs silyl ylides to form alkenes.
Interesting Facts About the Wittig Reaction
Here are some intriguing facts about the Wittig reaction that highlight its importance and versatility.
- Georg Wittig shared the Nobel Prize in Chemistry with Herbert C. Brown, who was recognized for his work on organoboranes.
The Wittig Reaction's Impact
The Wittig reaction has revolutionized organic chemistry. This powerful method allows chemists to create alkenes with precision, making it invaluable in synthesis. Named after Georg Wittig, who won a Nobel Prize for his work, this reaction has paved the way for countless advancements in pharmaceuticals, materials science, and agrochemicals.
Understanding the mechanism and applications of the Wittig reaction can open doors to innovative solutions in various fields. From creating complex molecules to developing new drugs, the possibilities are endless. This reaction's versatility and efficiency make it a staple in laboratories worldwide.
Whether you're a student, researcher, or just curious about chemistry, the Wittig reaction is a fascinating topic. Its impact on science and industry underscores the importance of continued exploration and innovation in chemical reactions. Keep learning, experimenting, and pushing the boundaries of what's possible.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
