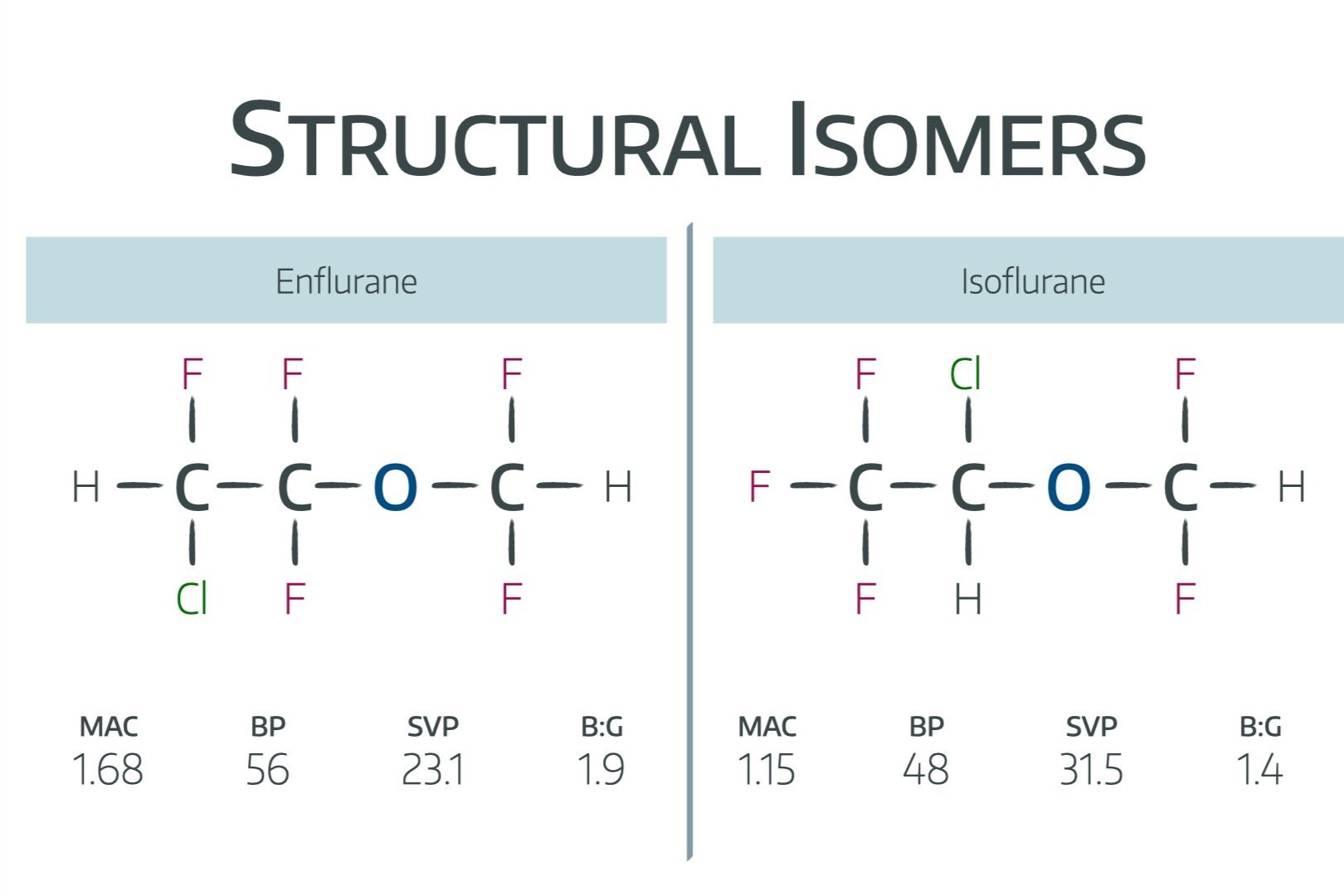
Structural isomers are fascinating molecules that share the same molecular formula but differ in the arrangement of their atoms. This difference can lead to unique properties and behaviors, making them a key topic in chemistry. Did you know that these isomers can be found in everything from simple hydrocarbons to complex biological molecules? Understanding structural isomers can help explain why certain substances have different smells, tastes, or even medicinal effects. In this blog post, we'll dive into 30 intriguing facts about structural isomers, shedding light on their importance in both everyday life and scientific research. Whether you're a student, a chemistry enthusiast, or just curious, these facts will give you a deeper appreciation for the diversity and complexity of molecules.
What Are Structural Isomers?
Structural isomers are fascinating molecules that share the same molecular formula but differ in the arrangement of their atoms. This unique characteristic leads to a variety of properties and behaviors in chemical reactions. Let's dive into some intriguing facts about these molecular marvels.
-
Structural isomers have the same molecular formula but different structures. This means that although they contain the same number of each type of atom, the atoms are connected in different ways.
-
There are three main types of structural isomers: chain isomers, position isomers, and functional group isomers. Each type has its own unique way of rearranging atoms.
-
Chain isomers differ in the arrangement of the carbon skeleton. For example, butane and isobutane both have the formula C4H10 but different carbon chain structures.
-
Position isomers have the same carbon skeleton but differ in the position of a functional group. An example is 1-butanol and 2-butanol, which both have the formula C4H10O but differ in the position of the hydroxyl group.
-
Functional group isomers contain different functional groups. For instance, ethanol (C2H6O) and dimethyl ether (C2H6O) have the same molecular formula but different functional groups (alcohol and ether, respectively).
Why Structural Isomers Matter
Understanding structural isomers is crucial in chemistry because their different structures lead to different chemical and physical properties. This can have significant implications in various fields, from pharmaceuticals to materials science.
-
Structural isomers can have vastly different boiling and melting points. For example, n-butane has a boiling point of -0.5°C, while isobutane boils at -11.7°C.
-
They can exhibit different reactivity. For instance, the reactivity of 1-butanol and 2-butanol in chemical reactions can vary due to the position of the hydroxyl group.
-
Structural isomers can have different densities. This property is crucial in applications where the density of a substance is a key factor.
-
They can also have different solubility in water and other solvents. This affects how they are used in various chemical processes.
-
In pharmaceuticals, structural isomers can have different biological activities. One isomer might be an effective drug, while another could be inactive or even harmful.
Examples of Structural Isomers in Everyday Life
Structural isomers are not just a topic for chemistry textbooks; they are present in many substances we encounter daily.
-
Glucose and fructose are structural isomers. Both have the formula C6H12O6 but differ in the arrangement of atoms, leading to different properties and uses.
-
Ethanol and dimethyl ether are structural isomers with different uses. Ethanol is commonly found in alcoholic beverages, while dimethyl ether is used as a propellant and refrigerant.
-
Acetone and propanal are structural isomers. Acetone is a common solvent, while propanal is used in the synthesis of other chemicals.
-
Butane and isobutane are used as fuels. Despite having the same molecular formula, their different structures make them suitable for different applications.
-
Ibuprofen and its isomers. Ibuprofen, a common pain reliever, has structural isomers that can affect its efficacy and safety.
How Structural Isomers Are Identified
Chemists use various techniques to identify and differentiate structural isomers. These methods help in understanding their properties and potential applications.
-
Mass spectrometry can differentiate isomers based on their fragmentation patterns. This technique helps identify the structure of unknown compounds.
-
Nuclear magnetic resonance (NMR) spectroscopy provides detailed information about the structure of isomers. It can reveal the arrangement of atoms within a molecule.
-
Infrared (IR) spectroscopy can identify functional groups in isomers. Different functional groups absorb IR radiation at different wavelengths.
-
Chromatography can separate isomers based on their physical and chemical properties. This technique is useful in both analytical and preparative chemistry.
-
X-ray crystallography can determine the three-dimensional structure of crystalline isomers. This method provides precise information about the arrangement of atoms.
Interesting Facts About Structural Isomers
Here are some more intriguing tidbits about structural isomers that highlight their complexity and importance.
-
Structural isomers can exist in both organic and inorganic compounds. This versatility makes them a broad and important category in chemistry.
-
The number of possible structural isomers increases exponentially with the number of carbon atoms. For example, there are only two isomers of butane (C4H10) but 75 isomers of decane (C10H22).
-
Isomerism is a key concept in stereochemistry. While structural isomers differ in connectivity, stereoisomers differ in spatial arrangement.
-
Some structural isomers can interconvert under certain conditions. This process is known as tautomerization, common in compounds like keto-enol tautomers.
-
Isomerism plays a crucial role in the flavor and fragrance industry. Different isomers can have distinct smells and tastes.
-
Structural isomers can affect the color of compounds. For example, different isomers of a dye can produce different colors.
-
In biochemistry, isomers can influence enzyme activity. Enzymes may only recognize and catalyze reactions for specific isomers.
-
Isomerism is important in polymer chemistry. The properties of polymers can vary significantly depending on the arrangement of their monomer units.
-
In environmental science, isomers can have different environmental impacts. Some isomers may be more toxic or persistent in the environment than others.
-
Structural isomers are a key consideration in the design of new materials. Their unique properties can be harnessed to create materials with specific characteristics.
The Fascinating World of Structural Isomers
Structural isomers are a captivating aspect of chemistry. They share the same molecular formula but differ in how their atoms are arranged. This unique characteristic leads to varied properties and behaviors, making them essential in fields like pharmaceuticals, materials science, and biochemistry.
Understanding structural isomers helps us appreciate the complexity and diversity of chemical compounds. It also underscores the importance of molecular structure in determining a substance's properties and functions. From simple hydrocarbons to complex biomolecules, structural isomers play a crucial role in the natural and synthetic worlds.
Next time you encounter a chemical compound, remember that its structure might hide a fascinating story of isomerism. This knowledge not only enriches our understanding of chemistry but also opens doors to new discoveries and innovations. Structural isomers truly highlight the intricate beauty of the molecular world.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
