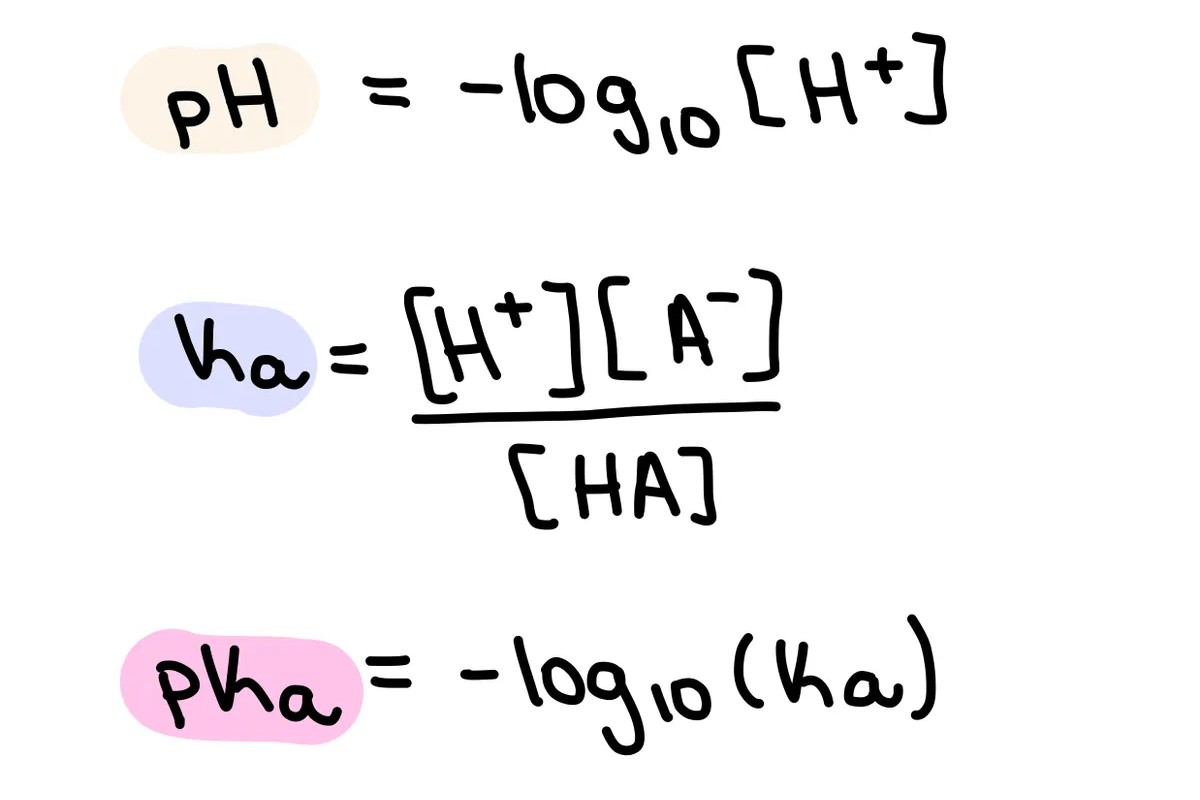
What is pKa? pKa stands for the acid dissociation constant, a measure of the strength of an acid in solution. It tells you how easily an acid can donate a proton (H⁺) to a base. A lower pKa value means a stronger acid because it dissociates more completely in water. This concept is crucial in chemistry, biology, and medicine, affecting everything from drug design to environmental science. Understanding pKa helps predict how molecules behave in different environments, which is essential for many scientific and industrial applications. Ready to dive into 30 intriguing facts about pKa? Let's get started!
What is pKa?
Understanding pKa is essential in chemistry, especially in acid-base reactions. It measures the strength of an acid in a solution. Here are some fascinating facts about pKa that will deepen your understanding.
-
pKa stands for "acid dissociation constant." It quantifies the tendency of an acid to donate a proton (H+).
-
The lower the pKa value, the stronger the acid. Strong acids have pKa values less than zero, while weak acids have higher pKa values.
-
pKa is logarithmic. A difference of one pKa unit represents a tenfold difference in acid strength.
-
Water has a pKa of 15.7. This means water is a very weak acid compared to strong acids like hydrochloric acid.
-
pKa is temperature-dependent. Changes in temperature can affect the pKa value of a substance.
Importance of pKa in Chemistry
pKa plays a crucial role in various chemical processes and reactions. It helps chemists predict the behavior of molecules in different environments.
-
Buffers rely on pKa. Buffers are solutions that resist changes in pH, and their effectiveness depends on the pKa of the acid/base pair used.
-
Drug design uses pKa values. Pharmaceutical chemists use pKa to predict how drugs will behave in the body.
-
Enzyme activity is influenced by pKa. The activity of enzymes can be affected by the pKa of amino acid residues in their active sites.
-
pKa helps in titration. Knowing the pKa of an acid or base helps determine the endpoint of a titration.
-
pKa affects solubility. The solubility of compounds can change with pH, which is influenced by their pKa values.
pKa in Everyday Life
pKa isn't just a concept for chemists; it has practical applications in everyday life.
-
Cooking involves pKa. The pKa of vinegar (acetic acid) affects its flavor and preservative qualities.
-
Cleaning products use pKa. The effectiveness of some cleaning agents depends on their pKa values.
-
pKa in beverages. The pKa of carbonic acid affects the fizziness of carbonated drinks.
-
pKa in skincare. The pKa of acids in skincare products influences their effectiveness and safety.
-
pKa in agriculture. Soil pH, which affects plant growth, is influenced by the pKa of various soil components.
Measuring pKa
Accurate measurement of pKa is vital for many scientific and industrial applications.
-
Potentiometric titration measures pKa. This method involves measuring the pH of a solution as a titrant is added.
-
Spectrophotometry can determine pKa. Changes in absorbance at different pH levels help calculate pKa values.
-
NMR spectroscopy measures pKa. Nuclear Magnetic Resonance (NMR) can provide information about the pKa of certain compounds.
-
Computational methods predict pKa. Advanced software can estimate pKa values based on molecular structure.
-
pKa can be measured in non-aqueous solvents. This is important for reactions that occur in organic solvents.
Interesting pKa Facts
Here are some intriguing tidbits about pKa that might surprise you.
-
Amino acids have multiple pKa values. Each amino acid has at least two pKa values, one for the carboxyl group and one for the amino group.
-
pKa affects protein folding. The pKa of amino acid side chains can influence the three-dimensional structure of proteins.
-
pKa and acid rain. The pKa of sulfuric and nitric acids contributes to the acidity of acid rain.
-
pKa in wine. The pKa of tartaric acid affects the taste and stability of wine.
-
pKa in the human body. The pKa of carbonic acid is crucial for maintaining blood pH.
Fun with pKa
Let's end with some fun and quirky facts about pKa.
-
pKa and spicy food. The pKa of capsaicin, the compound that makes chili peppers hot, affects its potency.
-
pKa in photography. The pKa of certain chemicals used in developing photos affects the process.
-
pKa in brewing. The pKa of acids in hops affects the bitterness of beer.
-
pKa in candy making. The pKa of citric acid influences the sourness of candies.
-
pKa in space. Scientists study the pKa of compounds in space to understand the chemistry of other planets.
The Final Word on pKa
Understanding pKa is crucial for anyone delving into chemistry. It helps predict how molecules behave in different environments, which is essential for fields like pharmacology, environmental science, and biochemistry. Knowing the pKa values of substances can guide you in creating better chemical reactions, developing new drugs, or even understanding natural processes.
Remember, pKa is all about the strength of an acid in a solution. Lower pKa means a stronger acid, while a higher pKa indicates a weaker one. This simple concept can unlock a deeper understanding of chemical interactions.
Whether you're a student, a professional, or just curious, grasping pKa can open doors to new insights and applications. Keep exploring, keep questioning, and let your curiosity lead the way. Chemistry is a vast field, and pKa is just one piece of the puzzle. Happy learning!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
