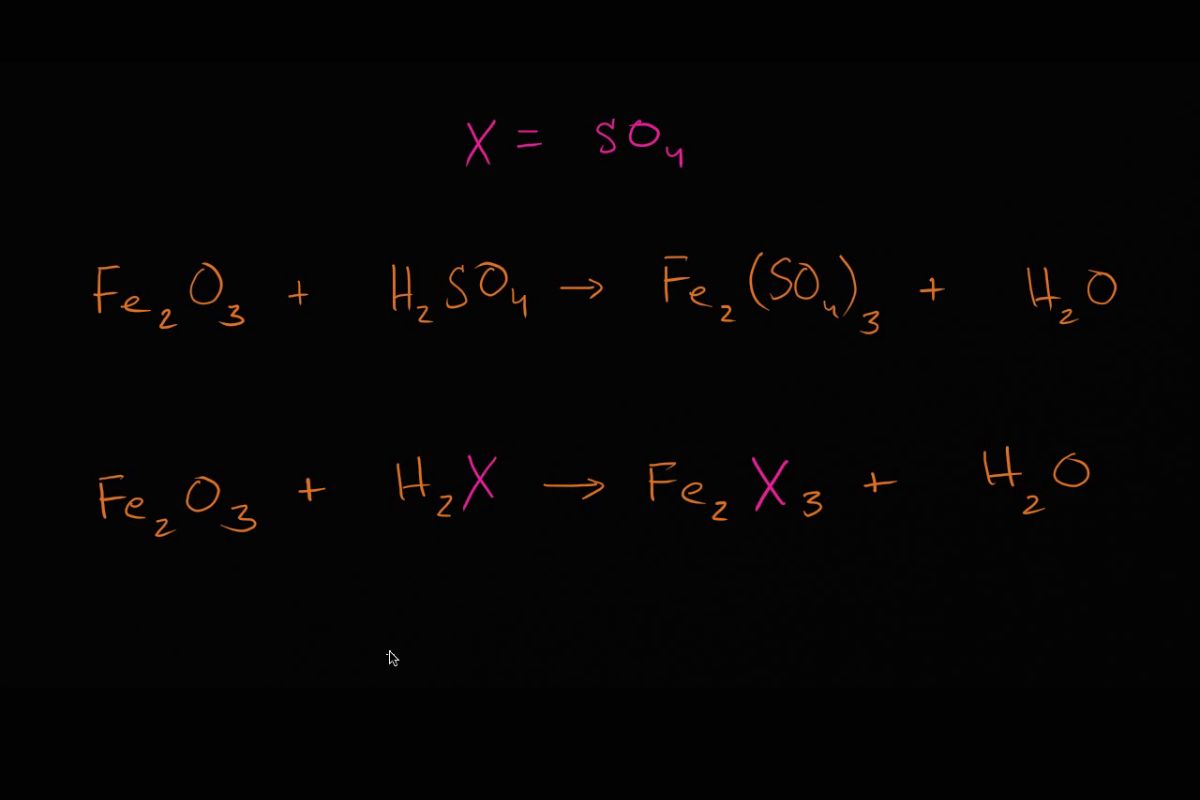
Balancing chemical equations might seem tricky, but it's a fundamental skill in chemistry. Why is balancing chemical equations important? Balancing chemical equations ensures that the same number of atoms for each element is present on both sides of the equation, following the Law of Conservation of Mass. This law states that matter cannot be created or destroyed in a chemical reaction. By balancing equations, chemists can accurately predict the amounts of reactants needed and products formed. This process helps in understanding reactions better, whether you're mixing baking soda and vinegar or studying complex biochemical pathways. Ready to dive into some fascinating facts about balancing chemical equations? Let's get started!
Understanding the Basics
Balancing chemical equations is a fundamental skill in chemistry. It ensures that the same number of atoms for each element is present on both sides of the equation. Here are some intriguing facts about this essential process.
-
Law of Conservation of Mass: Balancing chemical equations is based on the Law of Conservation of Mass, which states that mass cannot be created or destroyed in a chemical reaction.
-
Atoms Count: Each side of the equation must have the same number of atoms for each element involved.
-
Reactants and Products: Reactants are the starting substances, while products are the substances formed as a result of the reaction.
-
Coefficients: Numbers placed before compounds in an equation, called coefficients, are used to balance the equation.
-
Subscripts: Subscripts indicate the number of atoms in a molecule and cannot be changed to balance an equation.
Techniques and Tips
Balancing equations can be tricky, but several techniques can make the process easier. Here are some helpful tips.
-
Start with Single Elements: Begin by balancing elements that appear in only one reactant and one product.
-
Balance Polyatomic Ions as Units: If a polyatomic ion remains unchanged on both sides, balance it as a unit.
-
Save Hydrogen and Oxygen for Last: These elements often appear in multiple compounds, so balance them after other elements.
-
Use Fractional Coefficients: Sometimes, using fractions can simplify the balancing process. Multiply through by the denominator to clear fractions at the end.
-
Check Your Work: Always double-check that the number of atoms for each element is equal on both sides.
Real-World Applications
Balancing chemical equations isn't just an academic exercise. It has real-world applications in various fields.
-
Environmental Science: Helps in understanding and controlling pollution by balancing reactions involved in pollutant formation and degradation.
-
Pharmaceuticals: Essential for creating the correct proportions of ingredients in drug formulations.
-
Industrial Processes: Used in manufacturing processes to ensure the correct amounts of reactants are used to produce desired products efficiently.
-
Biochemistry: Vital for understanding metabolic pathways and reactions within living organisms.
-
Energy Production: Crucial in balancing reactions in combustion engines and power plants to optimize fuel use and reduce emissions.
Common Challenges
Even seasoned chemists can face challenges when balancing equations. Here are some common issues and how to overcome them.
-
Complex Equations: Multi-step reactions can be daunting. Break them down into simpler steps.
-
Multiple Products: When reactions produce multiple products, balance one product at a time.
-
Redox Reactions: These involve electron transfer and can be tricky. Use the half-reaction method to balance them.
-
Combustion Reactions: Often involve hydrocarbons and oxygen, producing carbon dioxide and water. Balance carbon and hydrogen first, then oxygen.
-
Incomplete Reactions: Sometimes, not all reactants convert to products. Account for this by including all possible products and reactants.
Fun Facts
Balancing chemical equations can also be fascinating and fun. Here are some interesting tidbits.
-
Historical Roots: The concept dates back to Antoine Lavoisier in the 18th century, who is considered the father of modern chemistry.
-
Educational Tools: Many online tools and apps can help students practice balancing equations interactively.
-
Puzzle-Like Nature: Some people enjoy balancing equations as a mental exercise, similar to solving puzzles.
-
Art and Science: Balancing equations requires both logical thinking and creativity, blending art and science.
-
Global Importance: Understanding chemical equations is crucial for scientific communication worldwide, transcending language barriers.
Advanced Concepts
For those who want to dive deeper, advanced concepts in balancing chemical equations offer more challenges and insights.
-
Stoichiometry: This involves calculating the quantities of reactants and products in a chemical reaction, based on balanced equations.
-
Limiting Reactants: Identifying the reactant that limits the amount of product formed is crucial in many chemical processes.
-
Yield Calculations: Balancing equations helps in calculating theoretical and actual yields of products in reactions.
-
Equilibrium Reactions: Some reactions reach a state of equilibrium where reactants and products are formed at the same rate. Balancing these equations involves understanding dynamic processes.
-
Isotopic Labeling: Used in research to trace the path of atoms through a reaction, requiring precise balancing of equations to interpret results accurately.
The Final Word on Balancing Chemical Equations
Balancing chemical equations might seem tricky at first, but with practice, it becomes second nature. Remember, the key is ensuring the number of atoms for each element is the same on both sides of the equation. This keeps the law of conservation of mass intact. Start by balancing elements that appear in only one reactant and one product, then move to more complex ones. Don’t forget to adjust coefficients, not subscripts.
Using these tips, you’ll find balancing equations less daunting and more like solving a puzzle. Whether you’re a student, a teacher, or just someone curious about chemistry, mastering this skill opens up a deeper understanding of how reactions work. Keep practicing, stay patient, and soon enough, you’ll balance equations with ease. Happy balancing!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
