Flame tests have been used for centuries to identify and analyze chemical elements. This unique technique involves heating a sample in a flame and observing the distinct color emitted. The phenomenon behind flame tests lies in the emission of light by excited atoms when they return to their ground state. These tests not only help in the identification of elements but also provide valuable insights into their electronic structure and properties.
Intriguingly, flame tests have unveiled some mind-blowing facts that showcase the wonders of chemistry. From vibrant colors to hidden elements, the world of flame tests never fails to amaze. In this article, we will delve into ten fascinating facts about flame tests that will ignite your curiosity and deepen your understanding of this remarkable field of chemistry.
Key Takeaways:
- The Flame Test is a cool way to identify elements based on the colors they emit when heated. It’s like a colorful fingerprint for each element, and it’s been used since ancient times!
- The Flame Test isn’t just for chemistry labs – it’s used in fireworks, art, and even cooking! It’s like a secret code that helps us identify elements and create beautiful, colorful experiences.
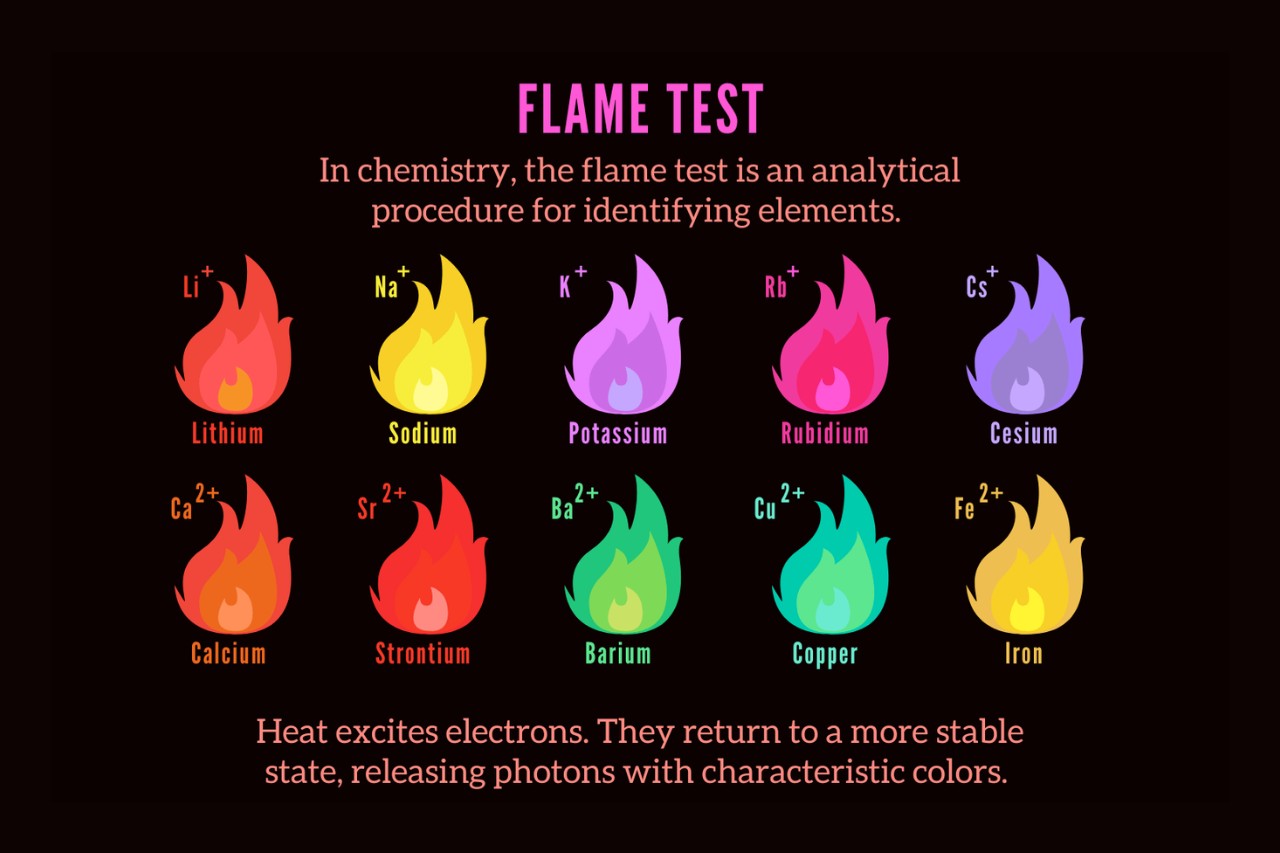
The Flame Test is a widely used analytical technique.
One of the most fascinating aspects of chemistry is the ability to identify and analyze different elements. The Flame Test is a commonly employed method for detecting and identifying elements based on the colors they emit when placed in a flame.
Different elements produce distinct colors in the Flame Test.
Each element has its unique electronic configuration, leading to the emission of specific wavelengths of light. This results in a characteristic color flame when the element is heated. For example, potassium produces a violet flame, while copper gives off a greenish-blue flame.
The Flame Test can be used to identify unknown substances.
The principle behind the Flame Test is that each element emits a specific color when heated, allowing scientists to identify unknown substances based on the color of the flame they produce. This is incredibly useful in various fields, from forensic science to environmental analysis.
The Flame Test is not limited to metals.
Although the Flame Test is commonly associated with metallic elements, non-metallic compounds can also display characteristic colors when exposed to a flame. For example, boron compounds produce a green flame, while sulfur-containing compounds yield a blue flame.
The Flame Test dates back to ancient times.
The concept of the Flame Test can be traced back to ancient civilizations such as the Chinese and Egyptians. They used flame colors to identify certain elements, even though they didn’t have a deep understanding of the underlying scientific principles.
The Flame Test is based on the energy levels of electrons.
When an element is heated in a flame, its electrons absorb energy and jump to higher energy levels. As these electrons return to their original energy levels, they release energy in the form of light, resulting in the characteristic color emission observed in the Flame Test.
The Flame Test is influenced by other factors.
Although the Flame Test is a valuable technique, the observed color can be affected by factors such as the concentration of the element, the temperature of the flame, and the presence of other compounds. These factors need to be taken into account for accurate interpretation.
The Flame Test is used in the field of pyrotechnics.
The vibrant colors seen in fireworks are achieved through the use of different metallic compounds. The Flame Test helps pyrotechnicians choose the right compounds to create the desired color effects in fireworks, adding an extra element of excitement to celebrations.
The Flame Test is an engaging educational experiment.
The Flame Test is often performed in high school and college chemistry labs as a hands-on activity to teach students about atomic structure, electron energy levels, and spectroscopy. It brings the theoretical concepts to life and sparks curiosity among students.
The Flame Test has applications beyond the laboratory.
Besides its scientific uses, the Flame Test has found applications in various fields. It is used in the art world to authenticate paintings, in the gemstone industry to identify impurities, and even in the culinary field to create visually appealing food presentations.
As you can see, the Flame Test is not only a fundamental analytical technique in chemistry but also a fascinating subject that has impacted various areas of our lives. Explore the 10 Mind-Blowing Facts About Flame Test to gain a deeper understanding of this colorful and captivating scientific method.
Conclusion
The flame test is a fascinating method used in chemistry to identify the presence of specific elements in a compound. The mesmerizing colorful glow emitted by different elements when subjected to heat is truly mind-blowing. By observing the unique colors produced during a flame test, scientists can determine the composition of unknown substances and gain valuable insights into the world of chemistry.
From the vibrant green flame of copper to the deep red flame of lithium, the flame test opens up a whole new dimension of understanding the elemental makeup of substances. This technique has been widely used for centuries and continues to play a significant role in various fields, including forensic science and pharmaceutical research.
Exploring the diverse range of colors generated by different elements can be both educational and awe-inspiring. Whether you’re a chemistry enthusiast or simply curious about the wonders of science, the flame test is sure to leave you spellbound.
FAQs
1. How does the flame test work?
The flame test works by exposing a sample to a flame, causing the electrons in the element to become excited. As the electrons return to their ground state, they emit light energy in the form of colorful flames.
2. Can any element be identified using the flame test?
No, not all elements produce distinct colors in the flame test. While some elements produce characteristic colors, others may not show visible color changes.
3. Is the flame test used only in chemistry labs?
No, the flame test is used in various fields, including forensic science, where it helps identify substances found at crime scenes. It is also used in industries such as pyrotechnics and fireworks manufacturing.
4. Are there any safety precautions to consider when conducting a flame test?
Yes, safety precautions should always be taken when working with open flames and chemical compounds. It is essential to wear appropriate protective gear and conduct the experiment in a well-ventilated area.
5. Can the flame test be used to determine the concentration of an element in a compound?
No, the flame test is primarily used to identify the presence of an element in a compound, not to determine its concentration. Other analytical techniques, such as spectrometry, are often employed for quantitative analysis.
The flame test is just one of many fascinating aspects of chemistry. Dive deeper into the world of elements, compounds, and reactions with our captivating chemistry facts. For those interested in the analytical side of things, our article on analytical chemistry delves into the techniques used to identify and quantify substances. And if you're curious about how metal ions interact with other molecules, our piece on chelates will surely pique your interest. Explore the wonders of chemistry and discover the secrets behind the science that shapes our world.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
