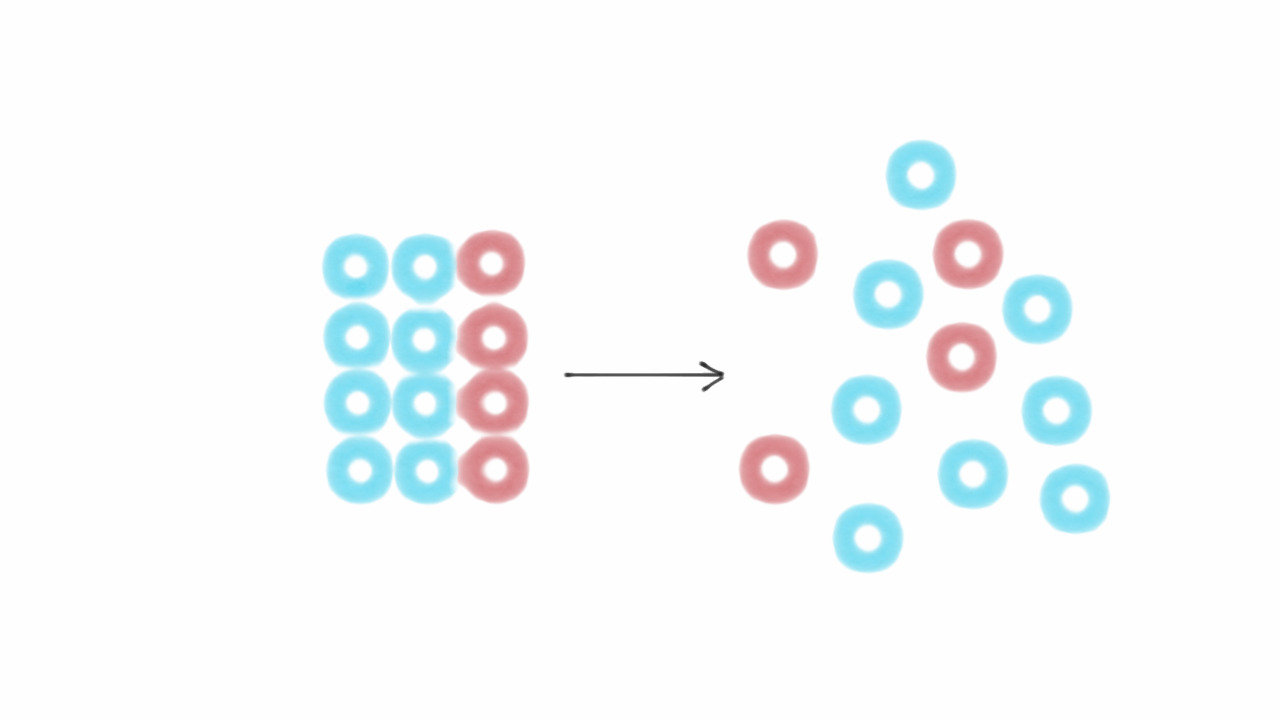
Ever wondered how refrigerators keep your food cold or how air conditioners cool your home? The magic lies in the Joule–Thomson effect. This fascinating phenomenon explains how gases change temperature when they expand or compress without exchanging heat with their surroundings. Named after James Prescott Joule and William Thomson, this effect plays a crucial role in various cooling technologies. Understanding it can help you grasp why certain gases cool down while others heat up during expansion. Whether you're a science enthusiast or just curious about everyday appliances, these 30 facts about the Joule–Thomson effect will enlighten you on the hidden science behind cooling systems.
What is the Joule–Thomson Effect?
The Joule–Thomson effect describes how a gas changes temperature when it expands without exchanging heat with its surroundings. This phenomenon plays a crucial role in various applications, from air conditioning to natural gas processing. Let's dive into some fascinating facts about this effect.
-
Named after physicists James Prescott Joule and William Thomson, who first studied it in the 1850s.
-
The effect occurs because gas molecules do work on each other during expansion, altering their internal energy.
-
Not all gases cool upon expansion; hydrogen and helium actually warm up under certain conditions.
-
The Joule–Thomson coefficient determines whether a gas will cool or heat during expansion.
-
This coefficient varies with temperature and pressure, making the effect complex to predict.
Applications of the Joule–Thomson Effect
The Joule–Thomson effect finds use in many practical applications. Understanding these uses can help appreciate its importance in everyday life.
-
Used in refrigerators and air conditioners to cool air by expanding refrigerant gases.
-
Essential in liquefying gases like oxygen and nitrogen for industrial and medical purposes.
-
Helps in natural gas processing to remove impurities and separate components.
-
Employed in cryogenics to achieve extremely low temperatures for scientific research.
-
Utilized in gas pipelines to control temperature and pressure, ensuring safe transport.
Factors Affecting the Joule–Thomson Effect
Several factors influence how the Joule–Thomson effect manifests. Knowing these can help in controlling and optimizing the effect for various applications.
-
Temperature: Higher initial temperatures can reduce the cooling effect during expansion.
-
Pressure: Greater pressure differences usually enhance the effect, making gases cool more.
-
Type of Gas: Different gases have unique Joule–Thomson coefficients, affecting their behavior.
-
Impurities: Presence of impurities can alter the gas's response to expansion.
-
Equipment Design: The design of expansion valves and other equipment can impact efficiency.
Historical Experiments and Discoveries
The history of the Joule–Thomson effect is filled with groundbreaking experiments and discoveries. These milestones have paved the way for modern applications.
-
James Joule and William Thomson first observed the effect in 1852 using a porous plug experiment.
-
Their work laid the foundation for the development of modern thermodynamics.
-
Early experiments involved air and carbon dioxide, revealing different behaviors.
-
The discovery of the inversion temperature, where the effect changes from cooling to heating, was a significant milestone.
-
Advances in technology have allowed for more precise measurements and control of the effect.
Modern Research and Developments
Ongoing research continues to uncover new aspects of the Joule–Thomson effect. These developments promise to enhance its applications and efficiency.
-
Researchers are exploring new materials for more efficient gas expansion.
-
Advances in computational modeling help predict the effect more accurately.
-
Studies on mixed gases aim to optimize industrial processes.
-
Innovations in cryogenics are pushing the boundaries of low-temperature applications.
-
Environmental considerations are driving research into more sustainable refrigerants.
Fun Facts and Trivia
The Joule–Thomson effect isn't just about serious science; it has some fun and quirky aspects too.
-
The effect is sometimes called the Joule-Kelvin effect, after William Thomson's later title, Lord Kelvin.
-
It plays a role in the operation of some types of gas masks.
-
The effect can be demonstrated with a simple experiment using a CO2 fire extinguisher.
-
Some high-altitude balloons use the effect to control internal temperature.
-
The Joule–Thomson effect even has applications in space exploration, helping to manage temperatures in spacecraft.
The Joule–Thomson Effect: A Quick Recap
The Joule–Thomson effect is a fascinating phenomenon in thermodynamics. It describes how a gas cools or heats when it expands without doing external work. This effect is crucial in various applications like refrigeration, air conditioning, and even in natural gas processing. Understanding this effect helps engineers design more efficient cooling systems and manage energy resources better.
Remember, the effect varies with different gases and temperatures. Some gases cool down, while others heat up during expansion. This behavior depends on the inversion temperature of the gas. Below this temperature, gases cool upon expansion; above it, they heat up.
Grasping the basics of the Joule–Thomson effect can provide valuable insights into everyday technologies and industrial processes. It’s a small but significant piece of the puzzle in the world of thermodynamics.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.