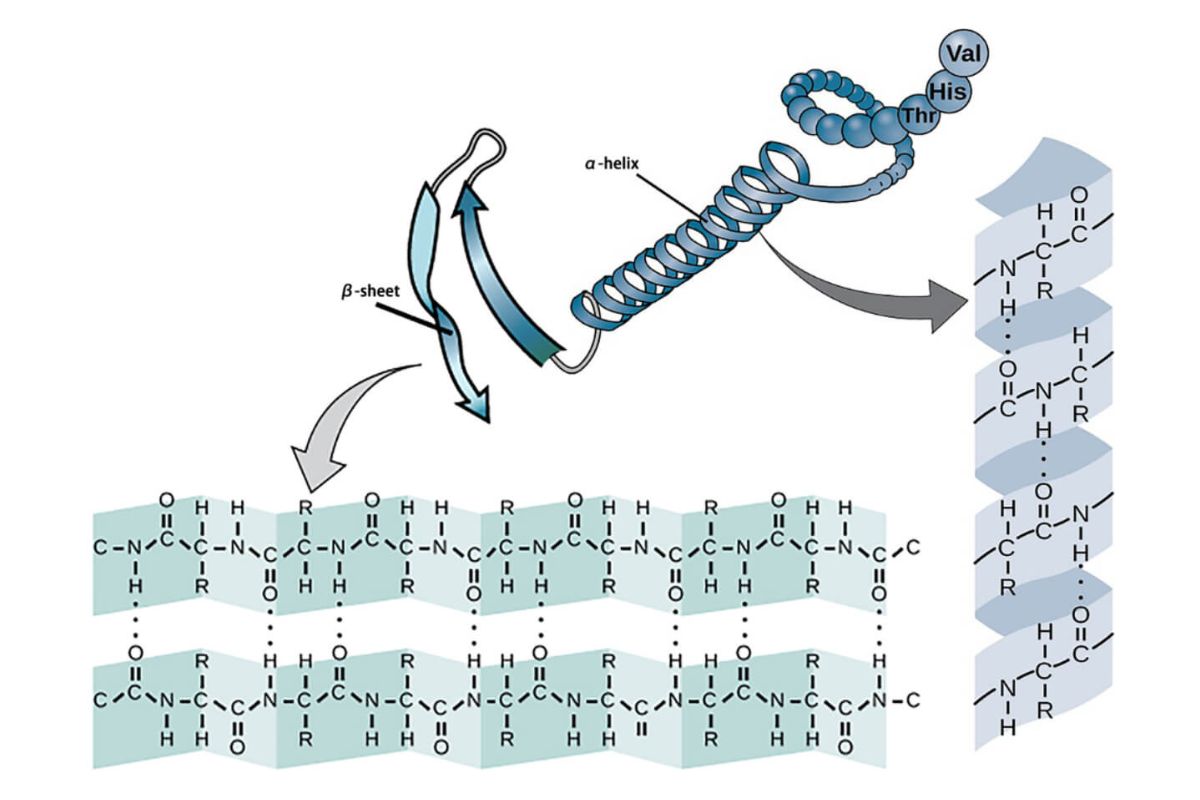
Secondary structure in proteins refers to the local folded shapes that form within a polypeptide due to hydrogen bonding. These structures include alpha helices and beta sheets, which are crucial for the protein's overall shape and function. Understanding these structures helps in fields like biochemistry, molecular biology, and medicine. Alpha helices resemble a coiled spring, while beta sheets look like folded paper. Both structures contribute to the protein's stability and ability to interact with other molecules. Knowing these facts can aid in grasping how proteins work, how they malfunction in diseases, and how new drugs can be designed.
What is Secondary Structure?
Secondary structure refers to the local folded structures that form within a polypeptide due to interactions between atoms of the backbone. These structures are crucial for the overall shape and function of proteins.
- Alpha Helix: A common secondary structure where the polypeptide chain coils into a spiral. Hydrogen bonds stabilize this structure.
- Beta Sheet: Another common form, consisting of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a sheet-like arrangement.
- Turns and Loops: These are non-repetitive secondary structures that connect alpha helices and beta sheets, allowing the protein to fold into its three-dimensional shape.
Importance of Secondary Structure
Understanding secondary structure is vital for comprehending how proteins function and interact within biological systems.
- Stability: Secondary structures provide stability to proteins, helping them maintain their shape under various conditions.
- Functionality: The specific arrangement of secondary structures determines the protein's function, such as enzyme activity or binding capabilities.
- Disease Association: Misfolding of secondary structures can lead to diseases like Alzheimer's and Parkinson's.
Alpha Helix Details
The alpha helix is one of the most studied secondary structures due to its prevalence and importance.
- Right-Handed Coil: Most alpha helices are right-handed, meaning they spiral in a clockwise direction.
- 3.6 Residues per Turn: Each turn of the helix contains approximately 3.6 amino acid residues.
- Hydrogen Bonding: Hydrogen bonds form between the carbonyl oxygen of one amino acid and the amide hydrogen of another, four residues away.
- R-Group Positioning: The side chains (R-groups) of the amino acids extend outward from the helix, reducing steric hindrance.
Beta Sheet Characteristics
Beta sheets are another fundamental secondary structure, playing a key role in the protein's overall architecture.
- Parallel and Antiparallel: Beta sheets can be parallel (strands run in the same direction) or antiparallel (strands run in opposite directions).
- Hydrogen Bonds: These bonds form between the carbonyl oxygen of one strand and the amide hydrogen of an adjacent strand.
- Pleated Structure: Beta sheets have a pleated or zigzag appearance due to the angles of the peptide bonds.
- Stability: Antiparallel beta sheets are generally more stable than parallel ones due to more optimal hydrogen bonding.
Turns and Loops
Turns and loops are flexible regions that connect more rigid secondary structures, allowing proteins to adopt complex shapes.
- Beta Turns: These are the most common type of turn, involving four amino acids and a hydrogen bond between the first and fourth residues.
- Gamma Turns: Less common, involving three amino acids and a hydrogen bond between the first and third residues.
- Role in Flexibility: Turns and loops provide the necessary flexibility for proteins to interact with other molecules.
- Surface Exposure: These regions are often found on the protein surface, making them accessible for interactions.
Techniques to Study Secondary Structure
Various techniques help scientists study and understand secondary structures in proteins.
- X-ray Crystallography: Provides detailed images of protein structures at atomic resolution.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Offers insights into protein structures in solution, capturing dynamic aspects.
- Circular Dichroism (CD) Spectroscopy: Measures the absorption of circularly polarized light to determine the content of alpha helices and beta sheets.
- Cryo-Electron Microscopy (Cryo-EM): Allows visualization of proteins in their native state without the need for crystallization.
Factors Influencing Secondary Structure
Several factors can influence the formation and stability of secondary structures in proteins.
- Amino Acid Sequence: The specific sequence of amino acids dictates the type of secondary structure that will form.
- Environmental Conditions: pH, temperature, and ionic strength can affect the stability of secondary structures.
- Post-Translational Modifications: Modifications like phosphorylation can alter the secondary structure and function of proteins.
- Chaperone Proteins: Assist in the proper folding of proteins, ensuring correct secondary structure formation.
Role in Protein Folding
Secondary structures are the building blocks of protein folding, leading to the formation of the final three-dimensional structure.
- Folding Pathways: Proteins follow specific pathways to fold into their functional forms, guided by secondary structures.
- Intermediate States: Secondary structures often form early in the folding process, serving as intermediates.
- Energy Landscape: The folding process is driven by the search for the lowest energy state, with secondary structures playing a key role.
Secondary Structure Prediction
Predicting secondary structure from amino acid sequences is a crucial aspect of bioinformatics.
- Algorithms: Various algorithms, like Chou-Fasman and GOR, predict secondary structures based on amino acid propensities.
- Machine Learning: Modern approaches use machine learning to improve prediction accuracy.
- Databases: Databases like PDB provide valuable data for training and validating prediction methods.
Applications in Biotechnology
Understanding secondary structures has numerous applications in biotechnology and medicine.
- Drug Design: Knowledge of secondary structures aids in designing drugs that target specific protein regions.
- Protein Engineering: Manipulating secondary structures allows scientists to create proteins with new functions.
- Disease Treatment: Targeting misfolded proteins and correcting their secondary structures can lead to new treatments for diseases.
Evolutionary Perspective
Secondary structures provide insights into the evolutionary history of proteins.
- Conserved Structures: Certain secondary structures are highly conserved across different species, indicating their importance.
- Homologous Proteins: Proteins with similar secondary structures often share a common ancestor.
- Functional Divergence: Changes in secondary structures can lead to new protein functions over evolutionary time.
Future Directions
Research on secondary structures continues to evolve, with new discoveries and technologies emerging.
- Advanced Imaging: Techniques like single-molecule FRET and super-resolution microscopy offer new ways to study secondary structures.
- Synthetic Biology: Designing synthetic proteins with specific secondary structures holds promise for various applications, from medicine to materials science.
Final Thoughts on Secondary Structures
Secondary structures play a crucial role in the world of biology. They form the backbone of proteins, determining their shape and function. Without these structures, proteins wouldn't be able to perform their essential tasks in our bodies. From alpha helices to beta sheets, each type of secondary structure has unique properties that contribute to the overall stability and functionality of proteins.
Understanding these structures helps scientists develop new medicines, improve agricultural practices, and even create innovative materials. The study of secondary structures isn't just for biologists; it has far-reaching implications in various fields. By grasping the basics, anyone can appreciate the complexity and beauty of life's building blocks.
So next time you hear about proteins, remember the importance of secondary structures. They may be small, but their impact is enormous. Keep exploring, stay curious, and never underestimate the power of these tiny yet mighty structures.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
