Peptide bonds are the unsung heroes of biology, holding together the very proteins that make life possible. But what exactly are they? Peptide bonds form when the carboxyl group of one amino acid links with the amino group of another, releasing a molecule of water in the process. This bond is crucial for creating the long chains of amino acids that fold into functional proteins. Without these bonds, our bodies couldn't build or repair tissues, produce enzymes, or even support basic cellular functions. Understanding peptide bonds can offer insights into everything from nutrition to genetic engineering. Ready to dive into 40 fascinating facts about these tiny but mighty connectors? Let's get started!
What Are Peptide Bonds?
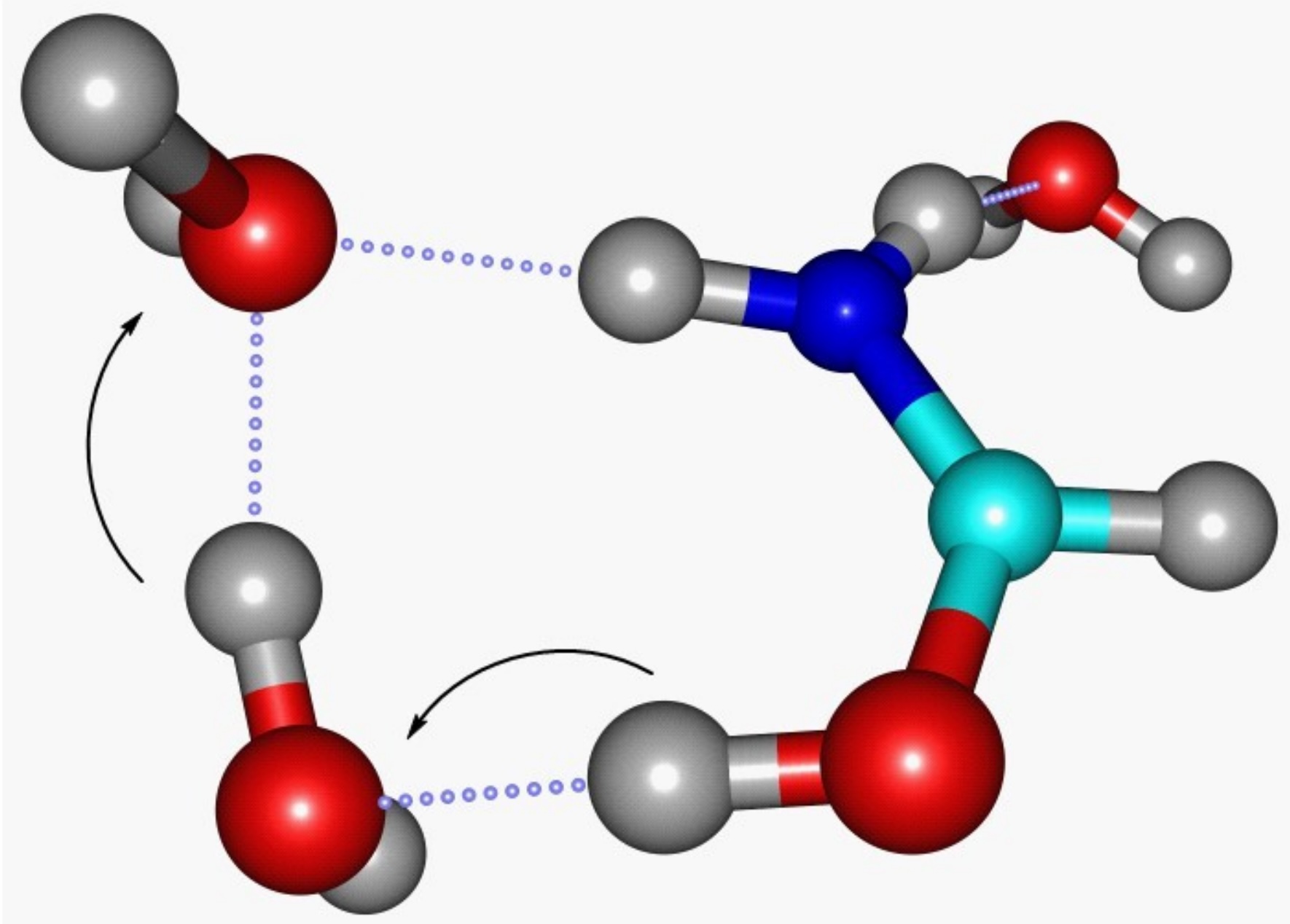
Peptide bonds are essential for life. They link amino acids together to form proteins, which are crucial for various biological functions. Here are some fascinating facts about these tiny but mighty bonds.
- Peptide bonds form between the carboxyl group of one amino acid and the amino group of another.
- This bond is a type of covalent bond, meaning it involves the sharing of electron pairs between atoms.
- The process of forming a peptide bond releases a molecule of water, a reaction known as a dehydration synthesis or condensation reaction.
- Peptide bonds are incredibly strong and stable, which is vital for the structural integrity of proteins.
- Breaking a peptide bond requires a significant amount of energy, often provided by enzymes in biological systems.
How Peptide Bonds Are Formed
Understanding the formation of peptide bonds helps us grasp their importance in protein synthesis. Let's dive into the details.
- Ribosomes in cells are the machinery that facilitates peptide bond formation during protein synthesis.
- Transfer RNA (tRNA) molecules bring amino acids to the ribosome, where peptide bonds are formed.
- The formation of a peptide bond is catalyzed by an enzyme called peptidyl transferase.
- This enzyme is part of the ribosome's large subunit, highlighting the complexity of cellular machinery.
- Peptide bond formation is a stepwise process, adding one amino acid at a time to the growing polypeptide chain.
The Role of Peptide Bonds in Proteins
Proteins owe their structure and function to peptide bonds. Here's how these bonds contribute to protein properties.
- Peptide bonds create the primary structure of proteins, which is the linear sequence of amino acids.
- The sequence of amino acids determines how the protein will fold into its secondary and tertiary structures.
- Hydrogen bonds, ionic bonds, and disulfide bridges further stabilize the folded protein, but peptide bonds hold the primary structure together.
- The unique sequence of amino acids in a protein is encoded by the organism's DNA.
- Mutations in DNA can lead to changes in the amino acid sequence, potentially altering the protein's function.
Peptide Bonds in Everyday Life
Peptide bonds aren't just a topic for biologists; they have practical implications in our daily lives. Here are some examples.
- Enzymes, which are proteins, rely on peptide bonds to maintain their structure and function.
- Hormones like insulin are proteins that regulate various physiological processes, all thanks to peptide bonds.
- Collagen, a protein found in skin and connective tissues, owes its strength to peptide bonds.
- Many medications, such as antibiotics, target bacterial proteins by disrupting their peptide bonds.
- Peptide bonds are also crucial in the food industry, affecting the texture and nutritional value of proteins in our diet.
Peptide Bond Stability and Degradation
While peptide bonds are strong, they can be broken down under certain conditions. Let's explore how this happens.
- Hydrolysis is the process of breaking a peptide bond by adding a molecule of water.
- Enzymes called proteases catalyze the hydrolysis of peptide bonds in proteins.
- The stomach enzyme pepsin breaks down dietary proteins into smaller peptides by cleaving peptide bonds.
- In cells, proteasomes degrade damaged or unneeded proteins by hydrolyzing their peptide bonds.
- Extreme pH levels or high temperatures can also break peptide bonds, denaturing the protein.
Peptide Bonds in Biotechnology
Biotechnology leverages peptide bonds for various applications, from medicine to research. Here are some ways they're used.
- Synthetic peptides are created in labs for research and therapeutic purposes.
- Peptide-based vaccines are being developed to target specific pathogens.
- Peptide bonds are used in drug delivery systems to release medication at targeted sites in the body.
- Biotechnology companies engineer proteins with specific peptide sequences for industrial applications.
- Peptide bonds are crucial in the development of biosensors, which detect biological molecules.
Fun Facts About Peptide Bonds
Peptide bonds have some quirky and interesting aspects. Let's take a look at a few fun facts.
- The peptide bond has a partial double-bond character, making it rigid and planar.
- This rigidity restricts the rotation around the bond, influencing protein folding.
- Peptide bonds absorb ultraviolet light, which is used in protein quantification techniques.
- The discovery of peptide bonds dates back to the early 20th century, revolutionizing our understanding of biochemistry.
- Peptide bonds can form cyclic structures, known as cyclic peptides, which have unique properties and functions.
Peptide Bonds and Evolution
Peptide bonds have played a crucial role in the evolution of life on Earth. Here's how they contributed to the diversity of life.
- The ability to form peptide bonds allowed early life forms to create proteins, leading to more complex organisms.
- Evolutionary changes in DNA sequences led to diverse protein structures and functions, all linked by peptide bonds.
- Peptide bonds enabled the development of enzymes, which catalyze biochemical reactions essential for life.
- The evolution of multicellular organisms relied on proteins with specialized functions, all held together by peptide bonds.
- Peptide bonds continue to be a focal point in evolutionary biology, helping scientists understand the origins and development of life.
Peptide Bonds: The Glue of Life
Peptide bonds are the unsung heroes holding proteins together. These tiny links form the backbone of protein structures, enabling countless biological processes. Without them, life as we know it wouldn't exist. They connect amino acids in a precise sequence, creating the diverse proteins that perform essential functions in our bodies.
Understanding peptide bonds helps us grasp how proteins work, from muscle contraction to immune responses. They also play a crucial role in medical research and drug development. Scientists study these bonds to design better treatments for diseases and understand genetic disorders.
In essence, peptide bonds are fundamental to life. Their importance can't be overstated. Next time you think about proteins, remember the tiny bonds making it all possible. They truly are the glue of life, holding everything together in a delicate balance.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
