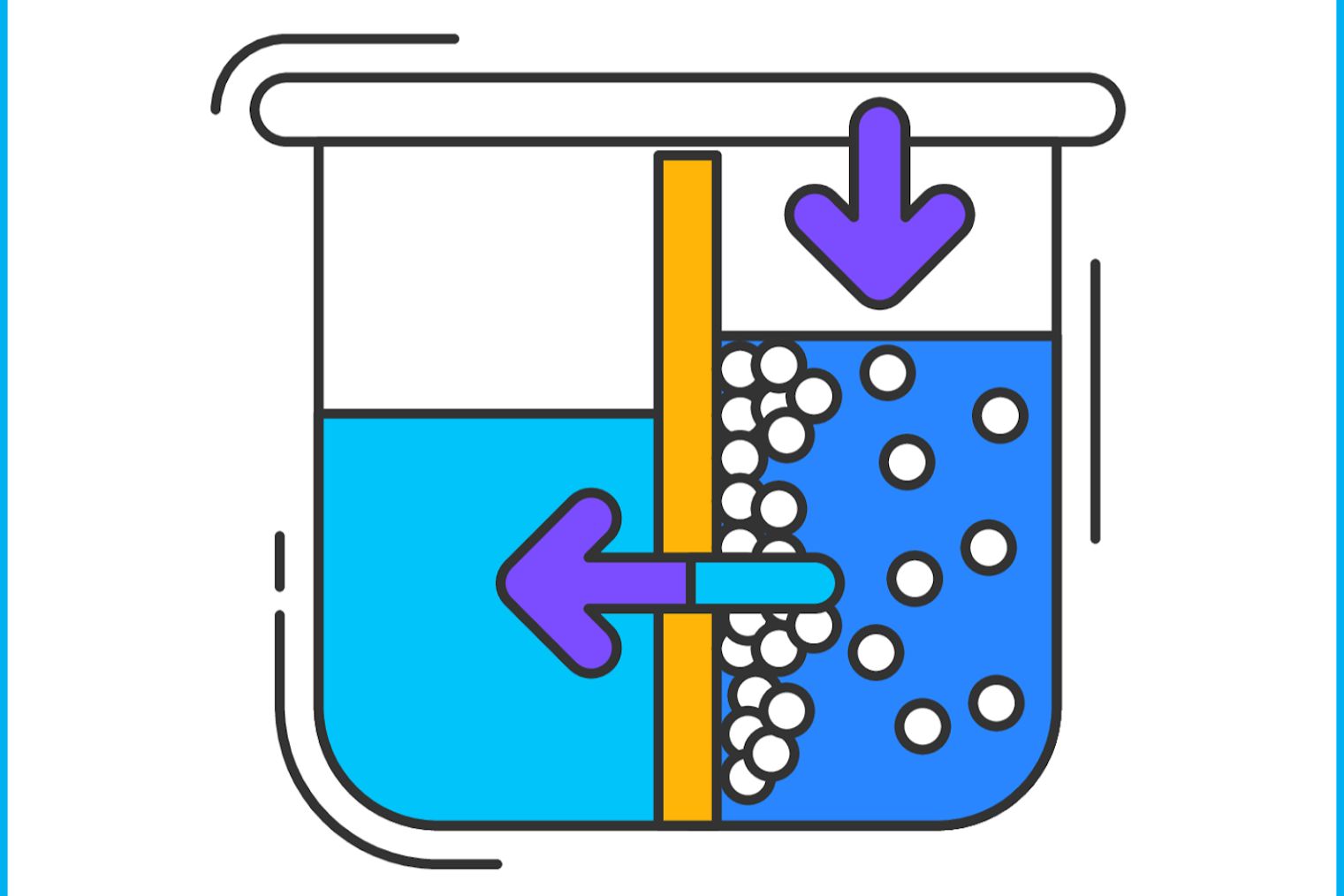
Osmotic pressure might sound like something out of a science fiction novel, but it's a real and fascinating concept in chemistry and biology. What is osmotic pressure? Osmotic pressure is the force caused by the movement of water through a semi-permeable membrane, driven by differences in solute concentration. This process is crucial for many biological functions, including nutrient absorption and waste removal in cells. Understanding osmotic pressure helps explain why plants stand tall, how kidneys filter blood, and even why you get thirsty after eating salty snacks. Dive into these 33 intriguing facts about osmotic pressure to see how this invisible force shapes life as we know it.
What is Osmotic Pressure?
Osmotic pressure is a fascinating concept in chemistry and biology. It describes the pressure required to stop the flow of water through a semipermeable membrane. This process is crucial for many biological functions and industrial applications.
- Osmotic pressure is the force needed to prevent water from moving across a semipermeable membrane.
- It plays a vital role in maintaining cell turgor, which keeps plant cells rigid.
- The concept was first introduced by Jacobus Henricus van 't Hoff, a Dutch physical chemist.
- Osmotic pressure can be calculated using the formula π = iMRT, where π is the osmotic pressure, i is the van 't Hoff factor, M is the molarity, R is the gas constant, and T is the temperature in Kelvin.
- Reverse osmosis, a process used to purify water, relies on osmotic pressure principles.
- Osmotic pressure is responsible for the movement of water in and out of cells, a process known as osmoregulation.
- In humans, kidneys use osmotic pressure to filter blood and produce urine.
- Osmotic pressure differences can cause cells to shrink (crenate) or swell (lyse) depending on the surrounding solution's concentration.
- It is a colligative property, meaning it depends on the number of solute particles in a solution, not their identity.
- Osmotic pressure helps plants absorb water from the soil through their roots.
How Osmotic Pressure Affects Everyday Life
Osmotic pressure isn't just a scientific concept; it impacts daily life in various ways. From food preservation to medical treatments, understanding osmotic pressure can be quite beneficial.
- Salting food preserves it by creating a high osmotic pressure environment that inhibits bacterial growth.
- Osmotic pressure is used in medical treatments like dialysis, where it helps remove waste products from the blood.
- It plays a role in the flavor and texture of pickled foods.
- Osmotic pressure is crucial in the production of certain beverages, like beer and wine, where it affects yeast activity.
- In agriculture, osmotic pressure influences irrigation practices and soil health.
- It is used in the cosmetic industry for products like moisturizers, which help maintain skin hydration.
- Osmotic pressure is a factor in the design of drug delivery systems, ensuring medications are released at the right rate.
- It affects the shelf life of many packaged foods by controlling moisture content.
- Osmotic pressure is essential in the brewing industry, impacting fermentation and flavor profiles.
- It helps in the desalination of seawater, making it a vital process for providing fresh water in arid regions.
Interesting Facts About Osmotic Pressure
Beyond its practical applications, osmotic pressure has some intriguing aspects that make it a captivating topic for science enthusiasts.
- The term "osmosis" comes from the Greek word "osmos," meaning "push" or "thrust."
- Osmotic pressure can be observed in everyday phenomena like the wilting of plants when they lack water.
- The osmotic pressure of seawater is about 27 atmospheres, which is why drinking seawater dehydrates you.
- Osmotic pressure can generate significant force; in some cases, it can burst cells if the pressure difference is too high.
- The study of osmotic pressure has led to advancements in understanding diseases like cystic fibrosis and diabetes.
- Osmotic pressure is a key factor in the survival of aquatic organisms, helping them maintain fluid balance.
- It is used in laboratories to study cell membranes and their permeability.
- Osmotic pressure differences are utilized in creating artificial organs and tissues.
- The phenomenon is essential in the food industry for processes like freeze-drying and concentration of juices.
- Osmotic pressure can be harnessed for energy production in a process known as pressure-retarded osmosis.
Fun Facts About Osmotic Pressure
Osmotic pressure isn't just about science and industry; it also has some fun and quirky aspects that make it an interesting topic for everyone.
- Some plants use osmotic pressure to move water and nutrients up from their roots to their leaves.
- Osmotic pressure can be demonstrated with a simple experiment using a potato, salt, and water.
- The concept of osmotic pressure has been used in science fiction, such as in stories involving space travel and life support systems.
Osmotic Pressure: The Final Word
Osmotic pressure is a fascinating concept that plays a crucial role in many biological and chemical processes. From keeping your cells hydrated to helping plants absorb water, it’s everywhere. Understanding how it works can give you a better grasp of everything from medical treatments to everyday phenomena like why your fingers get wrinkly in water.
Remember, osmotic pressure depends on the concentration of solutes in a solution. The higher the concentration, the greater the pressure. This principle is used in various applications, including water purification and food preservation.
So next time you see a plant standing tall or enjoy a glass of clean water, you’ll know a bit more about the science behind it. Osmotic pressure might seem complex, but its effects are all around us, making life as we know it possible.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
