Ever wondered how electrons move around the nucleus in an atom? The Sommerfeld Model might have the answers you're looking for. This model, an extension of the Bohr Model, dives deeper into atomic structure by introducing elliptical orbits and relativistic effects. It helps explain the fine structure of spectral lines, which Bohr's model couldn't. Developed by Arnold Sommerfeld in 1916, this theory was a significant step forward in quantum mechanics. Understanding the Sommerfeld Model can give you a clearer picture of atomic behavior and the complex dance of electrons. Ready to learn some intriguing facts about this groundbreaking model? Let's get started!
What is the Sommerfeld Model?
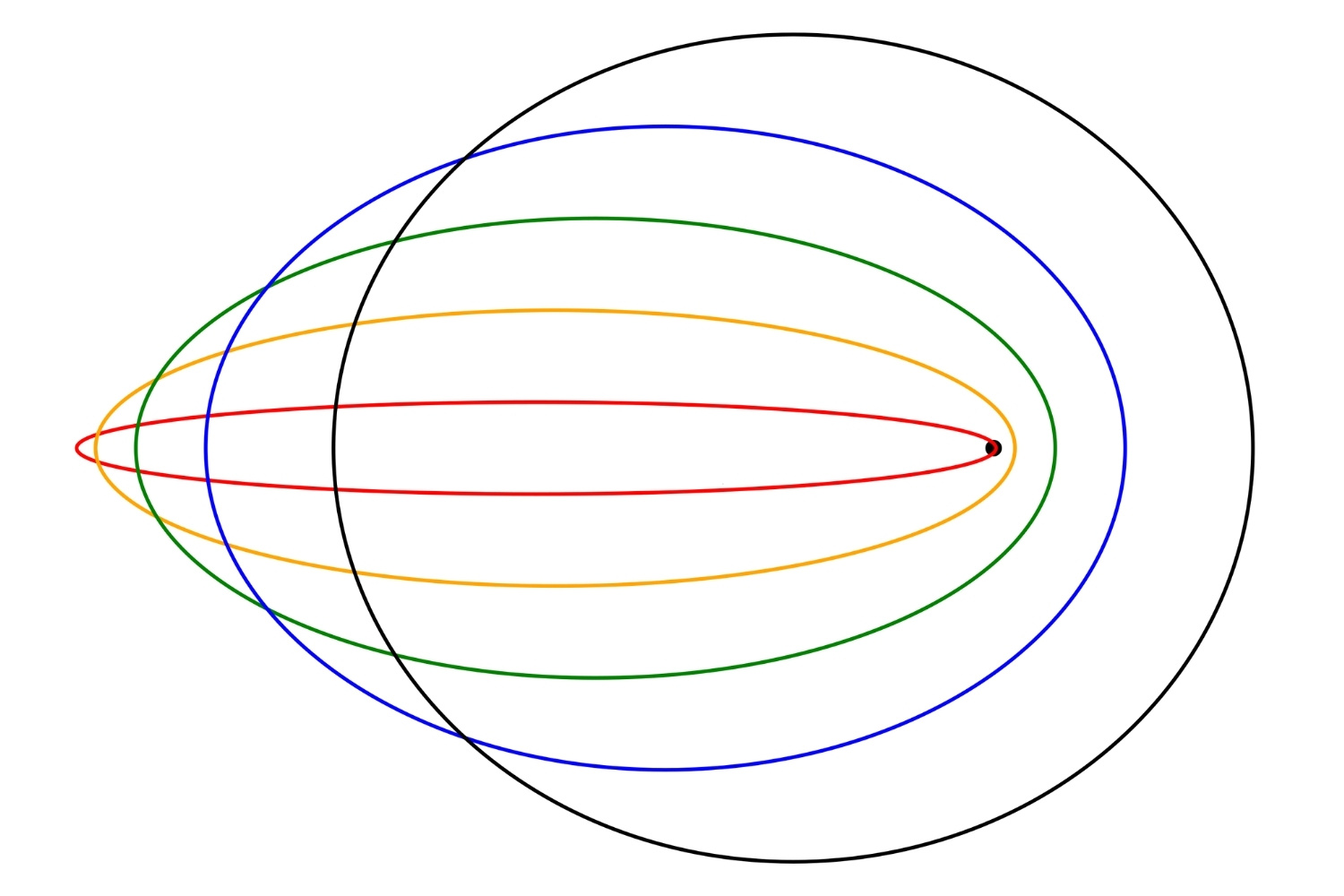
The Sommerfeld Model is an extension of the Bohr model of the atom. It was introduced by Arnold Sommerfeld in 1916 to address some of the limitations of Bohr's theory. This model incorporates elements of special relativity and elliptical orbits to provide a more accurate description of atomic structure.
- The Sommerfeld Model extends Bohr's model by including elliptical orbits for electrons, not just circular ones.
- It introduces the concept of azimuthal quantum numbers, which describe the shape of an electron's orbit.
- This model accounts for the fine structure of spectral lines, which Bohr's model couldn't explain.
- Sommerfeld's theory incorporates special relativity to correct for the high velocities of electrons in atoms.
- The model predicts the splitting of spectral lines into multiple components, known as fine structure splitting.
Key Components of the Sommerfeld Model
Understanding the Sommerfeld Model involves grasping several key components that differentiate it from Bohr's model. These components provide a more nuanced view of atomic behavior.
- Elliptical Orbits: Unlike Bohr's circular orbits, Sommerfeld's model allows for elliptical paths.
- Azimuthal Quantum Number (l): This number determines the shape of the electron's orbit.
- Magnetic Quantum Number (m): This number describes the orientation of the orbit in space.
- Relativistic Corrections: The model includes adjustments for the effects of special relativity on electron motion.
- Fine Structure Constant (α): A fundamental physical constant that characterizes the strength of the electromagnetic interaction between elementary charged particles.
Historical Context and Development
The development of the Sommerfeld Model didn't happen in isolation. It was part of a broader effort to refine atomic theory in the early 20th century.
- Arnold Sommerfeld introduced his model in 1916, building on Niels Bohr's earlier work.
- The model was developed to explain anomalies in the hydrogen spectrum that Bohr's model couldn't address.
- Sommerfeld's work was influenced by Albert Einstein's theory of special relativity.
- The model played a crucial role in the development of quantum mechanics.
- Sommerfeld's contributions earned him a reputation as one of the pioneers of atomic physics.
Applications and Implications
The Sommerfeld Model has had far-reaching implications in various fields of science and technology. Its applications extend beyond atomic physics.
- The model helps explain the fine structure of spectral lines in atomic spectra.
- It has applications in quantum chemistry, particularly in understanding electron configurations.
- The model is used in solid-state physics to describe the behavior of electrons in metals.
- It provides a foundation for the study of atomic collisions.
- Sommerfeld's theory has influenced the development of particle physics.
Limitations and Criticisms
Despite its advancements, the Sommerfeld Model is not without its limitations and criticisms. Understanding these helps in appreciating the evolution of atomic theory.
- The model doesn't fully account for electron-electron interactions.
- It fails to explain the hyperfine structure of spectral lines.
- The model is less effective for atoms with more than one electron.
- It doesn't incorporate the concept of electron spin, which was introduced later.
- The Sommerfeld Model has been largely superseded by quantum mechanics.
Legacy and Modern Relevance
Even though the Sommerfeld Model has been largely replaced by more advanced theories, its legacy endures in modern science.
- The model laid the groundwork for the development of quantum mechanics.
- It introduced concepts that are still used in modern atomic theory.
- Sommerfeld's work influenced many prominent physicists, including Werner Heisenberg and Wolfgang Pauli.
- The model is still taught in introductory courses on atomic physics.
- It serves as a historical example of the scientific method and the evolution of scientific theories.
Fun Facts About Arnold Sommerfeld
Arnold Sommerfeld was not just a brilliant physicist; he was also an interesting personality with a rich life story.
- Sommerfeld was a mentor to several Nobel Prize winners, including Wolfgang Pauli and Werner Heisenberg.
- He was known for his ability to explain complex concepts in simple terms.
- Sommerfeld had a passion for mountaineering and often drew analogies between climbing mountains and solving scientific problems.
Final Thoughts on the Sommerfeld Model
The Sommerfeld Model revolutionized our understanding of atomic structure. It built on Bohr's Model by introducing elliptical orbits and relativistic effects, providing a more accurate depiction of electron behavior. This model paved the way for quantum mechanics, influencing how scientists view atomic and subatomic particles today.
Understanding the Sommerfeld Model helps grasp the complexities of atomic physics and its applications in modern technology. From semiconductors to MRI machines, the principles derived from this model are foundational.
While newer models have emerged, the Sommerfeld Model remains a crucial milestone in the history of physics. It serves as a testament to human curiosity and the relentless pursuit of knowledge. Keep exploring, questioning, and learning; the world of science always has more to offer.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
