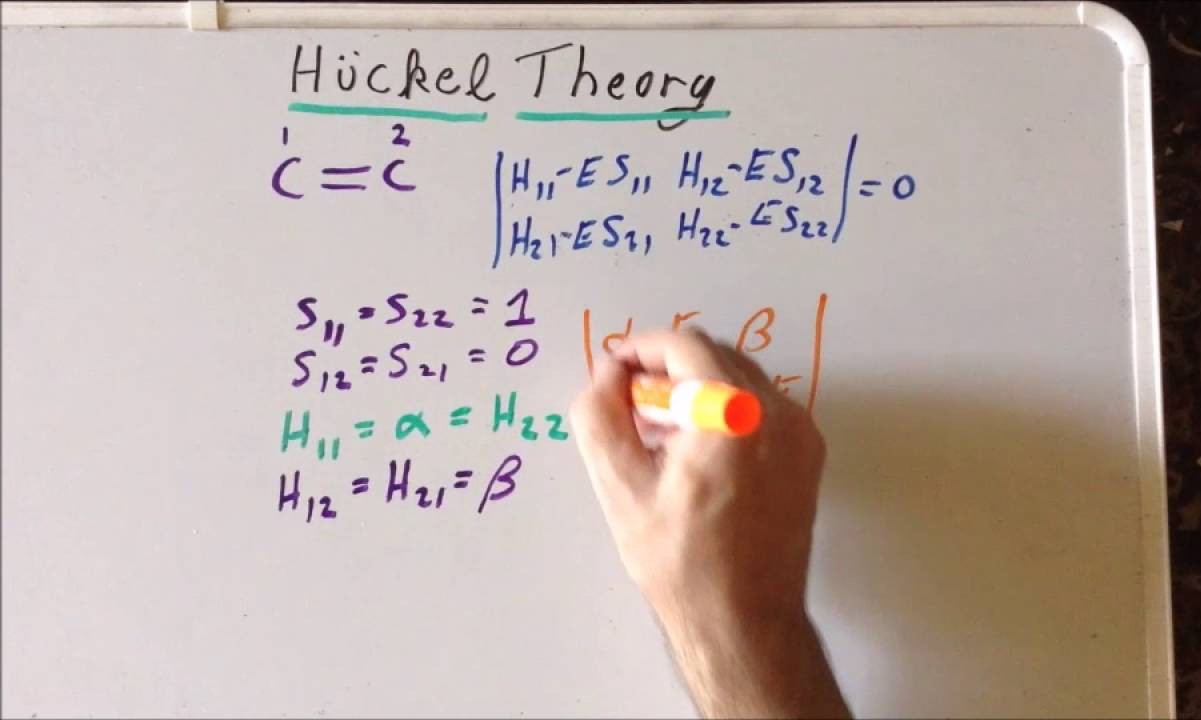
What is the Hückel Method? The Hückel Method is a simple way to understand the behavior of electrons in certain molecules, especially those with conjugated systems like benzene. Developed by Erich Hückel in the 1930s, this method uses quantum mechanics to predict molecular properties. It focuses on π-electrons in planar, cyclic molecules, helping chemists determine stability, reactivity, and electronic structure. Why is it important? Because it simplifies complex calculations, making it easier for students and researchers to grasp fundamental concepts in organic chemistry. Want to learn more? Here are 29 facts about the Hückel Method that will deepen your understanding.
What is the Hückel Method?
The Hückel method is a quantum chemistry technique used to determine the energies of molecular orbitals in conjugated systems. This method, named after German physicist Erich Hückel, simplifies complex calculations by focusing on π-electrons in planar molecules. Let's dive into some fascinating facts about this method.
-
Simplification of Schrödinger Equation: The Hückel method simplifies the Schrödinger equation for π-electrons, making it easier to solve for large molecules.
-
Planar Molecules: This method is specifically designed for planar, conjugated systems like benzene, where π-electrons are delocalized.
-
Hückel's Rule: According to Hückel's rule, a molecule is aromatic if it has (4n + 2) π-electrons, where n is a non-negative integer.
-
Matrix Representation: The method uses a matrix to represent the interactions between π-electrons, simplifying the calculations.
-
Eigenvalues and Eigenvectors: Solving the Hückel matrix gives eigenvalues and eigenvectors, which correspond to the energy levels and molecular orbitals.
Historical Background
Understanding the historical context of the Hückel method provides insight into its development and significance in chemistry.
-
Developed in 1931: Erich Hückel introduced this method in 1931, revolutionizing the study of aromatic compounds.
-
Inspired by Benzene: Hückel was inspired by the stability and unique properties of benzene, a molecule with six π-electrons.
-
Quantum Mechanics Era: The method emerged during the early days of quantum mechanics, a period of rapid advancements in theoretical chemistry.
-
Initial Skepticism: Initially, the scientific community was skeptical about the method's accuracy, but it gained acceptance over time.
-
Legacy: Hückel's work laid the foundation for modern computational chemistry, influencing many subsequent theories and methods.
Applications of the Hückel Method
The Hückel method has a wide range of applications in chemistry, particularly in understanding the properties of aromatic compounds.
-
Predicting Aromaticity: Chemists use the method to predict whether a molecule is aromatic, antiaromatic, or non-aromatic.
-
Molecular Orbital Theory: It helps in constructing molecular orbital diagrams, crucial for understanding chemical bonding.
-
Spectroscopy: The method aids in interpreting UV-Vis spectra of conjugated systems by predicting electronic transitions.
-
Chemical Reactivity: It provides insights into the reactivity of molecules, helping chemists design new reactions and compounds.
-
Polycyclic Aromatic Hydrocarbons: The method is particularly useful for studying polycyclic aromatic hydrocarbons (PAHs), which have multiple fused benzene rings.
Limitations of the Hückel Method
Despite its usefulness, the Hückel method has several limitations that chemists must consider.
-
Only π-Electrons: The method only considers π-electrons, ignoring σ-electrons, which can affect the accuracy of predictions.
-
Planarity Requirement: It is only applicable to planar molecules, limiting its use for non-planar systems.
-
Approximate Nature: The method provides approximate solutions, which may not be accurate for all molecules.
-
Neglect of Electron Correlation: It does not account for electron correlation effects, which can be significant in some cases.
-
Limited to Conjugated Systems: The method is not suitable for non-conjugated systems, restricting its applicability.
Advanced Concepts
For those interested in delving deeper, the Hückel method offers several advanced concepts worth exploring.
-
Extended Hückel Method: An extension of the original method, it includes σ-electrons and non-planar systems, providing more accurate results.
-
Hückel Molecular Orbital (HMO) Theory: This theory extends the method to more complex systems, including heteroatoms and substituted aromatics.
-
Computational Chemistry: Modern computational chemistry software often includes the Hückel method as a basic tool for molecular modeling.
-
Graph Theory: The method has connections to graph theory, where molecules are represented as graphs with vertices and edges.
-
Topological Indices: Chemists use topological indices derived from the Hückel method to predict molecular properties and activities.
Fun Facts
Let's end with some fun and lesser-known facts about the Hückel method.
-
Erich Hückel's Background: Hückel was originally a physicist, but his work had a profound impact on chemistry.
-
Nobel Prize: Although Hückel never won a Nobel Prize, his contributions are considered Nobel-worthy by many in the scientific community.
-
Educational Tool: The method is widely taught in undergraduate chemistry courses, serving as an introduction to quantum chemistry.
-
Cultural Impact: The Hückel method has even made its way into popular culture, being referenced in books and TV shows about science.
The Hückel Method's Impact
The Hückel method has revolutionized how chemists understand molecular orbitals and conjugated systems. By simplifying complex quantum mechanics, it offers a practical way to predict molecular behavior. This method has been pivotal in organic chemistry, helping scientists design new materials and drugs. Its simplicity and effectiveness make it a staple in chemical education.
Understanding the Hückel method opens doors to grasping more advanced theories and applications. It’s not just a tool for academics; it’s a bridge to real-world innovations. Whether you’re a student, researcher, or just curious about chemistry, knowing the basics of the Hückel method enriches your scientific knowledge.
So, next time you encounter a conjugated molecule, remember the Hückel method’s role in deciphering its secrets. It’s a testament to how simple ideas can lead to profound discoveries.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
