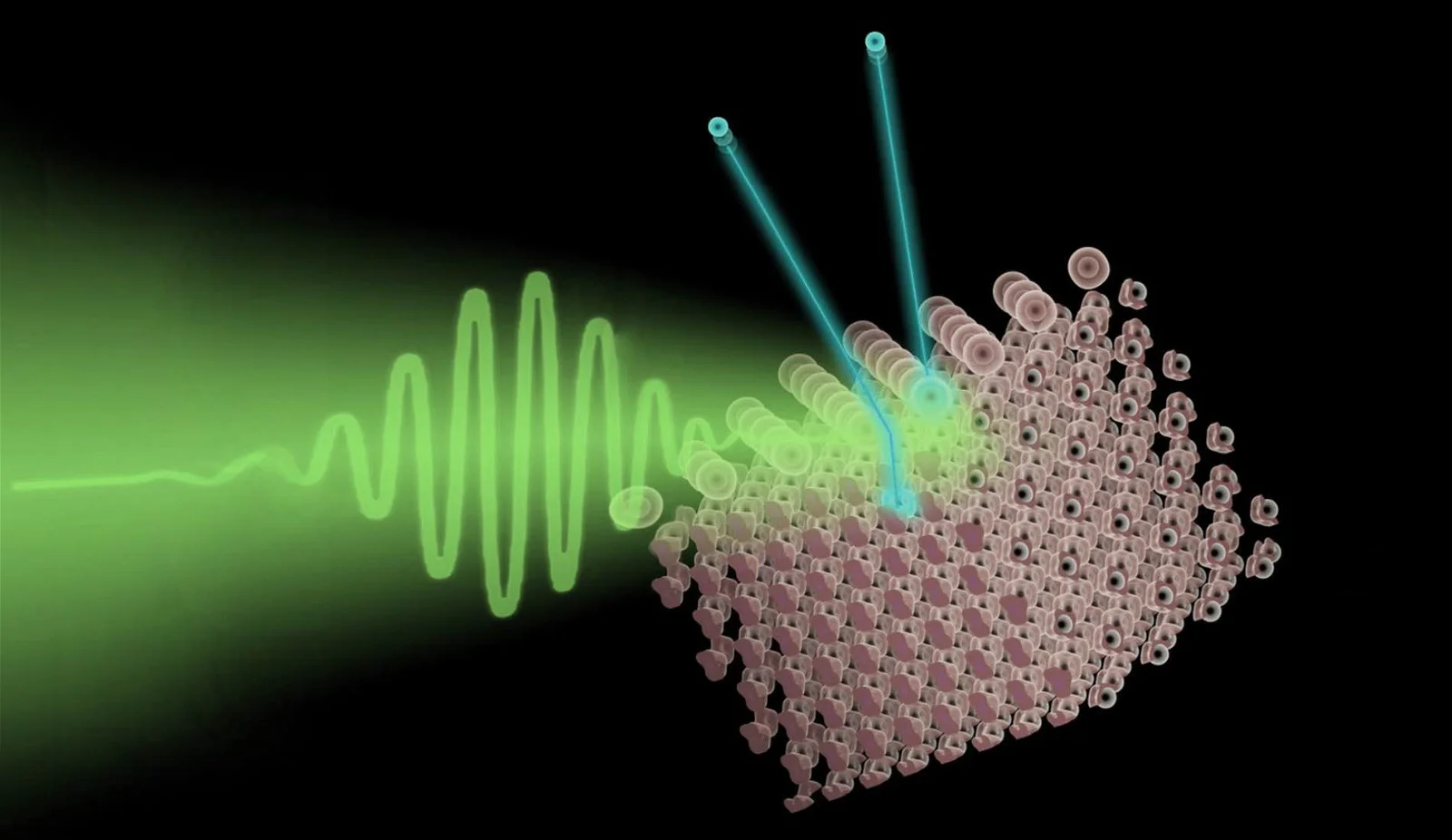
Photoemission is a fascinating phenomenon in the field of physics that has revolutionized our understanding of the behavior of light and the properties of matter. It involves the ejection of electrons from a material surface when it is exposed to light or other forms of electromagnetic radiation. This process, also known as the photoelectric effect, was first discovered by Heinrich Hertz in 1887 and later explained by Albert Einstein in 1905, establishing the foundation of modern physics.
In this article, we will delve into the intriguing world of photoemission and unveil 15 astounding facts that will expand your knowledge and appreciation for this remarkable scientific concept. From the discovery of the photoelectric effect to its applications in various fields, we will explore the intricate workings of photoemission and its profound impact on the world of science and technology.
Key Takeaways:
- Photoemission is the process of emitting electrons from a material when it’s exposed to light. It’s used in solar cells and electron microscopy, and it helped develop quantum mechanics.
- The energy of photoemitted electrons can be controlled through the use of light filters, and it has led to advances in surface science and quantum cryptography.
Photoemission is the process of emitting electrons from a material when it is exposed to light.
Photoemission, also known as the photoelectric effect, occurs when photons of light strike the surface of a material and transfer enough energy to electrons to overcome the binding forces holding them in place. This phenomenon was first explained by Albert Einstein in 1905.
The discovery of photoemission laid the foundation for the development of quantum mechanics.
Photoemission played a crucial role in the understanding of the particle-wave duality of light and electrons. It challenged the classical wave theory of light and led to the development of quantum mechanics, which revolutionized our understanding of the microscopic world.
Photoemission is utilized in the construction of solar cells.
Solar cells, also known as photovoltaic cells, rely on the principle of photoemission to convert sunlight into electricity. When photons from sunlight strike the surface of a solar cell, they cause the emission of electrons, which can then be captured and used as an electric current.
Photoemission is directly related to the work function of a material.
The work function is the minimum amount of energy required to remove an electron from the surface of a material. The ease with which photoemission occurs depends on the work function of the material. Lower work functions result in more efficient photoemission.
The photoelectric effect was initially observed using metals.
Early experiments on photoemission focused on metals, as they have low work functions and are therefore more easily photoemitted. However, it was later discovered that photoemission can occur in other materials as well, including semiconductors and insulators.
Photoemission can be used to determine the energy levels in a material.
By analyzing the energies of the emitted electrons, scientists can gain valuable insights into the electronic structure of a material. This information is extremely important in fields like solid-state physics and materials science.
The intensity of the incident light affects the number of photoemitted electrons.
The number of electrons emitted through photoemission is directly proportional to the intensity of the incident light. Higher intensity light results in a greater number of photoemitted electrons, while lower intensity light produces fewer electrons.
The photoemission process occurs almost instantly.
When photons strike the surface of a material, the photoemission process happens incredibly quickly, on the order of femtoseconds (10^-15 seconds). This prompt response allows for the efficient capture and utilization of the ejected electrons.
Different wavelengths of light have varying abilities to cause photoemission.
The photoemission process is most efficient when the incident light has a wavelength that matches the energy gap between the bound and unbound states of the electrons. Different materials have different energy gaps, resulting in variations in their photoemission responses.
The photoelectric effect was initially met with skepticism.
When the photoelectric effect was first proposed by Albert Einstein, it faced considerable skepticism from the scientific community. Many physicists at the time held on to the classical wave theory of light and found it difficult to accept the idea that light could behave as both a particle and a wave.
Photoemission is used in electron microscopy techniques.
Electron microscopy techniques, such as photoemission electron microscopy (PEEM) and scanning tunneling microscopy (STM), rely on the emission of electrons for imaging and analysis purposes. These techniques allow for high-resolution imaging of materials at the nanoscale.
The energy of photoemitted electrons can be controlled through the use of light filters.
By selectively filtering the incident light, it is possible to control the energy distribution of the emitted electrons. This manipulation of energy levels is significant in applications such as photoemission spectroscopy, where specific energy ranges need to be examined.
The study of photoemission has led to advances in surface science.
Photoemission has been instrumental in advancing the field of surface science, which focuses on understanding the properties and behavior of materials at their surfaces. The ability to analyze the electronic structure of surfaces has facilitated the development of better catalysts, sensors, and electronic devices.
The photoelectric effect has implications for quantum cryptography.
The principles underlying the photoelectric effect have been utilized in the field of quantum cryptography, where the transmission of information is secured by the properties of quantum mechanics. Photon detectors based on photoemission are used to detect individual photons and ensure secure communication.
Photoemission can be observed in various forms of light, including ultraviolet and X-rays.
While the photoelectric effect is commonly associated with visible light, it can also occur with other forms of electromagnetic radiation. Ultraviolet light and X-rays have higher energies than visible light and can cause photoemission in certain materials.
These 15 astounding facts about photoemission demonstrate the significance and diverse applications of this phenomenon. From its foundational role in quantum mechanics to its practical applications in solar cells and electron microscopy, photoemission continues to shape our understanding of light and matter.
Conclusion
In conclusion, photoemission is a fascinating phenomenon that plays a crucial role in various fields of physics and technology. It allows us to understand the behavior of electrons and their interaction with light. The principles of photoemission have led to significant advancements in areas such as solar energy, electron microscopy, and even everyday devices like digital cameras.By harnessing the power of photoemission, scientists have revolutionized our understanding of the quantum world. It has provided a profound understanding of concepts such as the photoelectric effect, work function, and energy bands in solids.From the discovery of the photoelectric effect by Albert Einstein to the development of ultrafast laser spectroscopy techniques, photoemission continues to push the boundaries of our knowledge and pave the way for exciting new discoveries. Its applications are vast and continue to evolve, promising a future filled with even more astounding breakthroughs in the world of physics.
FAQs
1. What is photoemission?
Photoemission is a phenomenon where electrons are emitted from a material’s surface when it is exposed to light or electromagnetic radiation.
2. What is the photoelectric effect?
The photoelectric effect refers to the emission of electrons from a material’s surface when illuminated by light of a certain frequency or above a threshold energy.
3. How does photoemission contribute to solar energy?
Photoemission plays a crucial role in the generation of electricity in photovoltaic cells. When light strikes the surface of a semiconductor material, it triggers photoemission, causing electrons to be released and creating a flow of electric current.
4. What are energy bands in solids?
Energy bands in solids refer to the distribution of allowed energy states for electrons in a material. Photoemission spectroscopy is commonly used to study the energy band structure of different materials.
5. How is photoemission used in electron microscopy?
Photoemission electron microscopy (PEEM) utilizes photoemission to create high-resolution images of a material’s surface at the nanoscale. By detecting the emitted electrons, PEEM provides valuable insights into the surface structure and composition of materials.
6. What are the future prospects of photoemission research?
The future of photoemission research holds great promise in advancing our understanding of fundamental physics and developing new technologies. It may lead to breakthroughs in areas such as quantum computing, advanced materials, and even novel sources of clean energy.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
