Solvation is a fascinating process where molecules of a solvent surround and interact with molecules or ions of a solute. This interaction plays a crucial role in many chemical reactions and processes. But what exactly happens during solvation? Why does solvation matter in everyday life and scientific research? Understanding solvation can help explain why certain substances dissolve in water while others do not, how medications work in the body, and even why some materials are better at conducting electricity. In this article, we'll explore 39 intriguing facts about solvation that will shed light on its importance and applications. Get ready to dive into the world of solvation and discover its many wonders!
What is Solvation?
Solvation is a fascinating process where solvent molecules surround and interact with solute ions or molecules. This interaction is crucial in many chemical reactions and processes. Let's dive into some intriguing facts about solvation.
-
Solvation vs. Hydration: Solvation refers to the general process of solvent molecules surrounding solute particles. When water is the solvent, the process is specifically called hydration.
-
Energy Changes: Solvation involves energy changes. The process can either absorb or release energy, depending on the nature of the solute and solvent.
-
Solvent-Solute Interaction: The strength of the interaction between solvent and solute molecules determines the solubility of the solute in the solvent.
Types of Solvation
Different types of solvation exist, each with unique characteristics. Understanding these types helps in various scientific and industrial applications.
-
Ion-Dipole Interaction: This type occurs when ionic compounds dissolve in polar solvents like water. The positive and negative ions are surrounded by the oppositely charged ends of the solvent molecules.
-
Dipole-Dipole Interaction: Involves polar molecules where the positive end of one molecule is attracted to the negative end of another.
-
Hydrogen Bonding: A special type of dipole-dipole interaction where hydrogen atoms bond with electronegative atoms like oxygen or nitrogen.
Importance of Solvation
Solvation plays a critical role in many natural and industrial processes. Its importance cannot be overstated.
-
Biological Systems: Solvation is essential in biological systems, affecting protein folding, enzyme activity, and DNA stability.
-
Pharmaceuticals: Drug solubility and bioavailability are influenced by solvation, impacting how medications are absorbed and utilized in the body.
-
Chemical Reactions: Many chemical reactions occur in solution, where solvation influences reaction rates and mechanisms.
Factors Affecting Solvation
Several factors can influence the solvation process. Understanding these factors helps in controlling and optimizing solvation in various applications.
-
Temperature: Higher temperatures generally increase solvation rates by providing more kinetic energy to the molecules.
-
Pressure: Changes in pressure can affect solvation, especially in gases. Higher pressure can increase the solubility of gases in liquids.
-
Nature of Solvent and Solute: Polar solvents dissolve polar solutes well, while non-polar solvents are better for non-polar solutes.
Real-World Applications
Solvation has numerous real-world applications that impact everyday life and various industries.
-
Cleaning Agents: Solvation is the principle behind how detergents and soaps clean. They dissolve dirt and grease, making them easier to wash away.
-
Batteries: In batteries, solvation helps in the movement of ions between electrodes, crucial for the battery's function.
-
Food Industry: Solvation affects the flavor and texture of food products. It plays a role in dissolving flavors and nutrients.
Fun Facts About Solvation
Let's explore some fun and lesser-known facts about solvation that might surprise you.
-
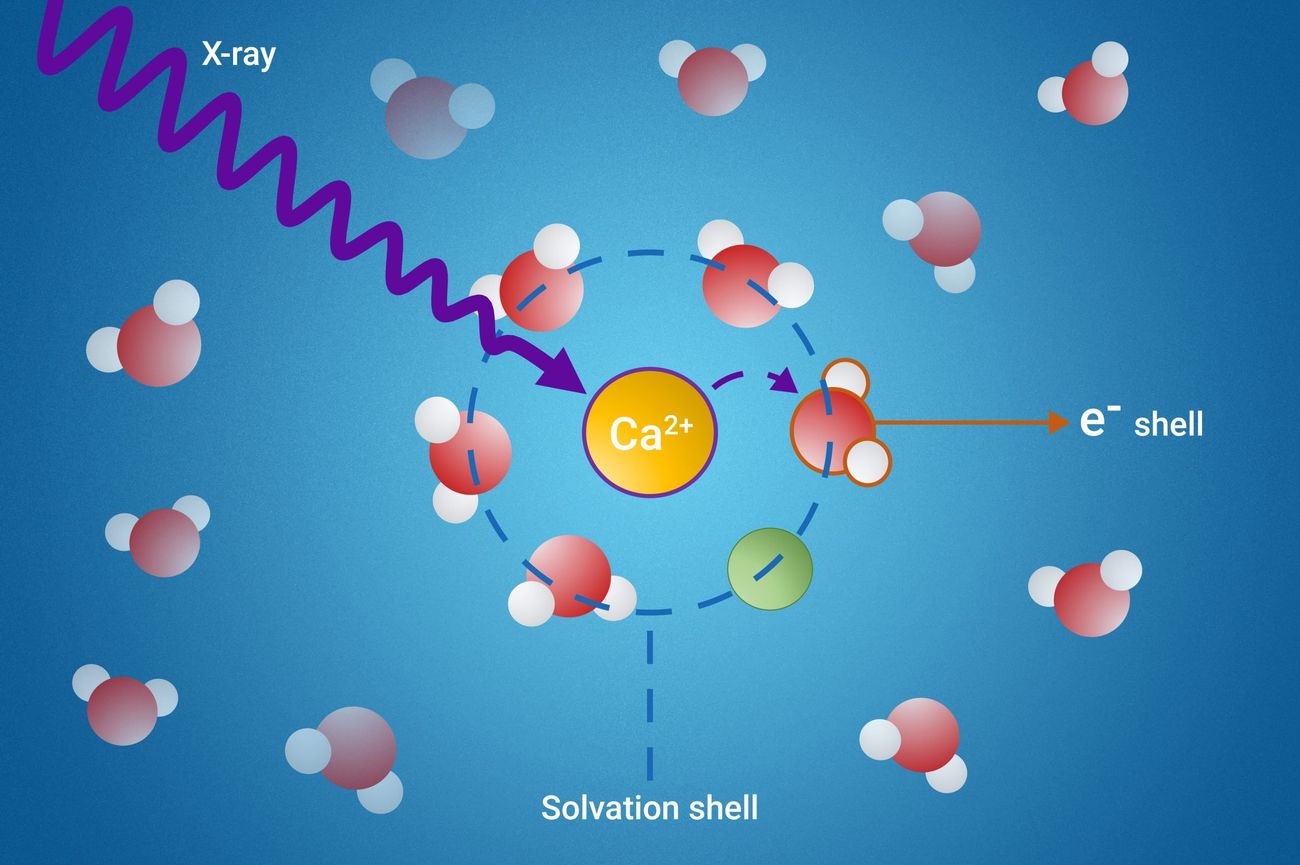
Solvation Shells: Solvent molecules form a "shell" around solute particles. This shell can have multiple layers, depending on the strength of the interaction.
-
Color Changes: Solvation can cause color changes in solutions. For example, when copper sulfate dissolves in water, it turns blue due to solvation.
-
Temperature Anomalies: Some solutes, like sodium acetate, dissolve better in hot water but crystallize out when cooled, a phenomenon used in hand warmers.
Historical Insights
The study of solvation has a rich history, with many scientists contributing to our understanding over the years.
-
Svante Arrhenius: This Swedish scientist proposed the theory of electrolytic dissociation, explaining how salts dissolve in water to form ions.
-
Wilhelm Ostwald: A pioneer in physical chemistry, Ostwald's work on solvation and solutions earned him a Nobel Prize in Chemistry in 1909.
-
Linus Pauling: Known for his work on chemical bonding, Pauling's research also contributed to understanding solvation and its effects on molecular structures.
Solvation in Everyday Life
Solvation isn't just a scientific concept; it affects many aspects of daily life.
-
Cooking: When you dissolve salt or sugar in water while cooking, you're witnessing solvation in action.
-
Beverages: The fizz in carbonated drinks is due to the solvation of carbon dioxide in water.
-
Personal Care: Many personal care products, like shampoos and lotions, rely on solvation to deliver active ingredients effectively.
Advanced Concepts in Solvation
For those interested in diving deeper, here are some advanced concepts related to solvation.
-
Solvation Dynamics: This field studies the time-dependent aspects of solvation, including how quickly solvation shells form and change.
-
Quantum Solvation: Involves studying solvation at the quantum level, where the behavior of electrons and nuclei in solute and solvent molecules is considered.
-
Solvation Free Energy: A measure of the energy change when a solute dissolves in a solvent, important in predicting solubility and reaction outcomes.
Environmental Impact
Solvation also has implications for the environment, influencing various natural processes.
-
Water Pollution: Solvation affects how pollutants dissolve and spread in water bodies, impacting water quality and aquatic life.
-
Soil Chemistry: Solvation influences nutrient availability in soil, affecting plant growth and soil health.
-
Atmospheric Chemistry: Solvation of gases in atmospheric water droplets plays a role in weather patterns and climate change.
Solvation in Technology
Modern technology leverages solvation in innovative ways, driving advancements in various fields.
-
Nanotechnology: Solvation helps in the stabilization and functionalization of nanoparticles, crucial for their application in medicine and electronics.
-
Material Science: Understanding solvation aids in designing better materials with desired properties, such as improved solubility and stability.
-
Energy Storage: Solvation processes are key in developing efficient energy storage systems, including advanced batteries and supercapacitors.
Challenges in Solvation Research
Despite its importance, solvation research faces several challenges that scientists are working to overcome.
-
Complexity: The solvation process can be highly complex, involving multiple interactions and dynamic changes that are difficult to study.
-
Measurement Techniques: Developing accurate and reliable techniques to measure solvation properties remains a challenge.
-
Predictive Models: Creating models that can accurately predict solvation behavior for different solute-solvent combinations is an ongoing area of research.
Future of Solvation Research
The future of solvation research holds exciting possibilities, with potential breakthroughs on the horizon.
-
Artificial Intelligence: AI and machine learning are being used to predict solvation properties and design new solvents with tailored characteristics.
-
Green Solvents: Research is focused on developing environmentally friendly solvents that can replace harmful ones in industrial processes.
-
Biomedical Applications: Advances in solvation research could lead to new drug delivery systems and therapies, improving healthcare outcomes.
The Final Word on Solvation
Solvation is a fascinating process where molecules or ions are surrounded by solvent molecules. This interaction plays a crucial role in many chemical reactions and biological processes. Understanding solvation helps scientists develop better pharmaceuticals, improve industrial processes, and even create new materials.
From the way water dissolves salt to how enzymes function in our bodies, solvation is everywhere. It affects everything from the taste of your food to the effectiveness of your medicine. Knowing these facts can give you a deeper appreciation for the science behind everyday phenomena.
So, next time you see sugar dissolving in your coffee or wonder how your body processes nutrients, remember solvation is at work. It's a small but mighty force shaping the world around us. Keep exploring and stay curious about the wonders of science!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
