Boiling point elevation is a fascinating phenomenon in chemistry. Ever wondered why adding salt to water makes it boil at a higher temperature? Boiling point elevation occurs when a non-volatile solute, like salt, is added to a solvent, such as water. This addition disrupts the solvent's ability to vaporize, requiring more heat to reach the boiling point. This principle is crucial in various applications, from cooking to industrial processes. Understanding boiling point elevation can help you grasp how solutions behave under different conditions. Ready to dive into 39 intriguing facts about this concept? Let's get started!
What is Boiling Point Elevation?
Boiling point elevation is a fascinating phenomenon in chemistry. It occurs when a solute is added to a solvent, causing the boiling point of the solvent to increase. This effect is a colligative property, meaning it depends on the number of solute particles rather than their identity.
- Boiling point elevation happens because the solute particles disrupt the formation of vapor bubbles in the solvent.
- The boiling point of a solution is always higher than that of the pure solvent.
- This effect is commonly observed when salt is added to water.
How Does Boiling Point Elevation Work?
Understanding the mechanics behind boiling point elevation can be quite intriguing. It involves the interaction between solute and solvent particles.
- When a solute dissolves, it creates a solution with a lower vapor pressure than the pure solvent.
- The lower vapor pressure means more heat is required to make the solvent boil.
- This additional heat results in a higher boiling point for the solution.
Factors Affecting Boiling Point Elevation
Several factors can influence the extent of boiling point elevation. These include the nature of the solute, the solvent, and the concentration of the solution.
- Ionic compounds like salt cause a more significant boiling point elevation compared to non-ionic compounds.
- The molecular weight of the solute can also affect the boiling point elevation.
- Higher concentration of solute particles leads to a greater increase in the boiling point.
Real-World Applications of Boiling Point Elevation
Boiling point elevation isn't just a lab curiosity; it has practical applications in everyday life and various industries.
- Cooking: Adding salt to water when cooking pasta increases the boiling point, cooking the pasta faster.
- Antifreeze: Ethylene glycol in car radiators raises the boiling point of the coolant, preventing overheating.
- Food preservation: Sugar added to jams and jellies raises the boiling point, helping to preserve them.
Calculating Boiling Point Elevation
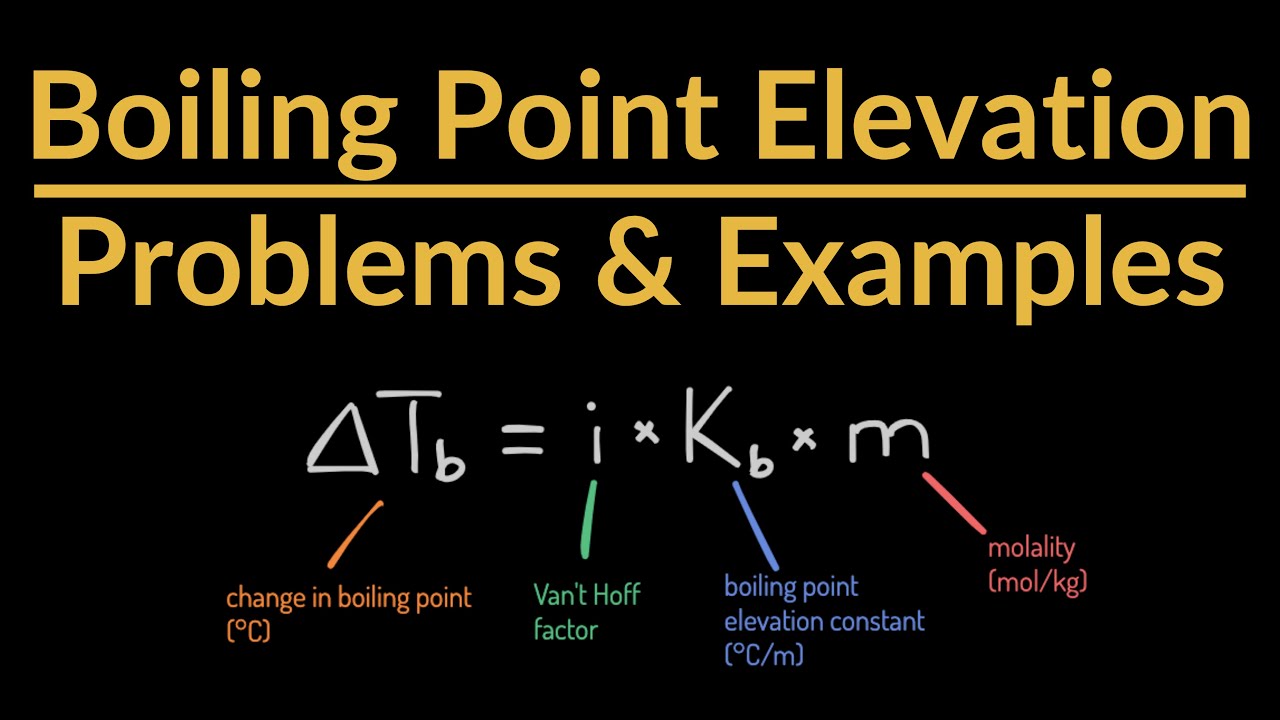
The calculation of boiling point elevation involves a straightforward formula, but understanding it can be quite rewarding.
- The formula is ΔTb = iKbm, where ΔTb is the boiling point elevation, i is the van 't Hoff factor, Kb is the ebullioscopic constant, and m is the molality.
- The van 't Hoff factor (i) represents the number of particles the solute breaks into.
- The ebullioscopic constant (Kb) is a property of the solvent and varies for different solvents.
Interesting Facts About Boiling Point Elevation
Here are some lesser-known yet intriguing facts about boiling point elevation that might surprise you.
- Seawater has a higher boiling point than freshwater due to dissolved salts.
- Pressure cookers utilize boiling point elevation by increasing pressure, which raises the boiling point of water inside.
- Altitude affects boiling point elevation; water boils at a lower temperature at higher altitudes, but adding solutes can counteract this effect.
Boiling Point Elevation in Nature
Nature provides several examples of boiling point elevation, showcasing its importance in various natural processes.
- Ocean water: The high salt content in oceans raises the boiling point, affecting marine life and weather patterns.
- Hot springs: Minerals dissolved in hot springs elevate the boiling point, contributing to their unique properties.
- Blood plasma: The presence of various solutes in blood plasma raises its boiling point, crucial for maintaining body temperature.
Historical Context of Boiling Point Elevation
The concept of boiling point elevation has a rich history, with significant contributions from various scientists.
- François-Marie Raoult first described the effect in the 19th century.
- Jacobus Henricus van 't Hoff expanded on Raoult's work, introducing the van 't Hoff factor.
- The study of boiling point elevation has led to advancements in thermodynamics and physical chemistry.
Boiling Point Elevation in Different Solvents
Different solvents exhibit varying degrees of boiling point elevation, depending on their properties.
- Water: One of the most studied solvents, with a well-known boiling point elevation effect.
- Ethanol: Shows a significant boiling point elevation when mixed with solutes.
- Benzene: Another solvent where boiling point elevation is observed, used in various chemical processes.
Boiling Point Elevation in Industrial Processes
Industries leverage boiling point elevation for various applications, enhancing efficiency and safety.
- Chemical manufacturing: Controlling boiling points is crucial for reactions and separations.
- Pharmaceuticals: Ensuring the stability of solutions by managing boiling points.
- Food processing: Utilizing boiling point elevation to improve cooking and preservation methods.
Boiling Point Elevation and Freezing Point Depression
Boiling point elevation is often discussed alongside freezing point depression, another colligative property.
- Both properties depend on the number of solute particles.
- Freezing point depression lowers the freezing point of a solution.
- These effects are used together in antifreeze formulations.
Boiling Point Elevation in Everyday Life
Everyday activities often involve boiling point elevation, even if we don't always notice it.
- Making tea: Adding sugar to tea raises its boiling point slightly.
- Laundry: Detergents can raise the boiling point of water, enhancing cleaning efficiency.
- Canning: Preserving food by boiling it in a solution with added solutes.
Fun Facts About Boiling Point Elevation
Some fun and quirky facts about boiling point elevation that might make you see this phenomenon in a new light.
- Boiling point elevation can be demonstrated with simple kitchen experiments.
- Astronauts need to consider boiling point elevation when cooking in space.
- Boiling point elevation plays a role in making candy, affecting the texture and consistency.
Boiling Point Elevation: The Final Word
Boiling point elevation is a fascinating phenomenon. When a non-volatile solute is added to a solvent, the boiling point of the solution increases. This happens because the solute particles disrupt the solvent molecules, making it harder for them to escape into the gas phase. The more solute added, the higher the boiling point becomes.
Understanding this concept is crucial in various fields, from cooking to industrial processes. For example, adding salt to water when cooking pasta raises the boiling point, allowing the pasta to cook at a slightly higher temperature. In industrial settings, controlling boiling points can be essential for chemical reactions and manufacturing processes.
Boiling point elevation is a simple yet powerful concept that affects everyday life and advanced scientific applications. Knowing how it works can help you make better decisions in the kitchen and understand more complex scientific principles.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
