Ever wondered what makes atoms tick? Understanding s, p, d, and f orbitals is key to grasping the basics of chemistry. These orbitals describe where electrons hang out around an atom's nucleus. s orbitals are spherical, while p orbitals look like dumbbells. d orbitals have more complex shapes, and f orbitals are even more intricate. Each type of orbital can hold a specific number of electrons, which affects how atoms bond and interact. Knowing these details can help you understand everything from why water is liquid to how fireworks get their colors. Ready to dive in? Let's explore 37 fascinating facts about these orbitals!
Understanding Atomic Orbitals
Atomic orbitals are regions around an atom's nucleus where electrons are likely to be found. These orbitals come in different shapes and sizes, each with unique properties. Let's dive into some fascinating facts about the s, p, d, and f orbitals.
s Orbitals
s orbitals are the simplest type of atomic orbitals. They have unique characteristics that set them apart from other orbitals.
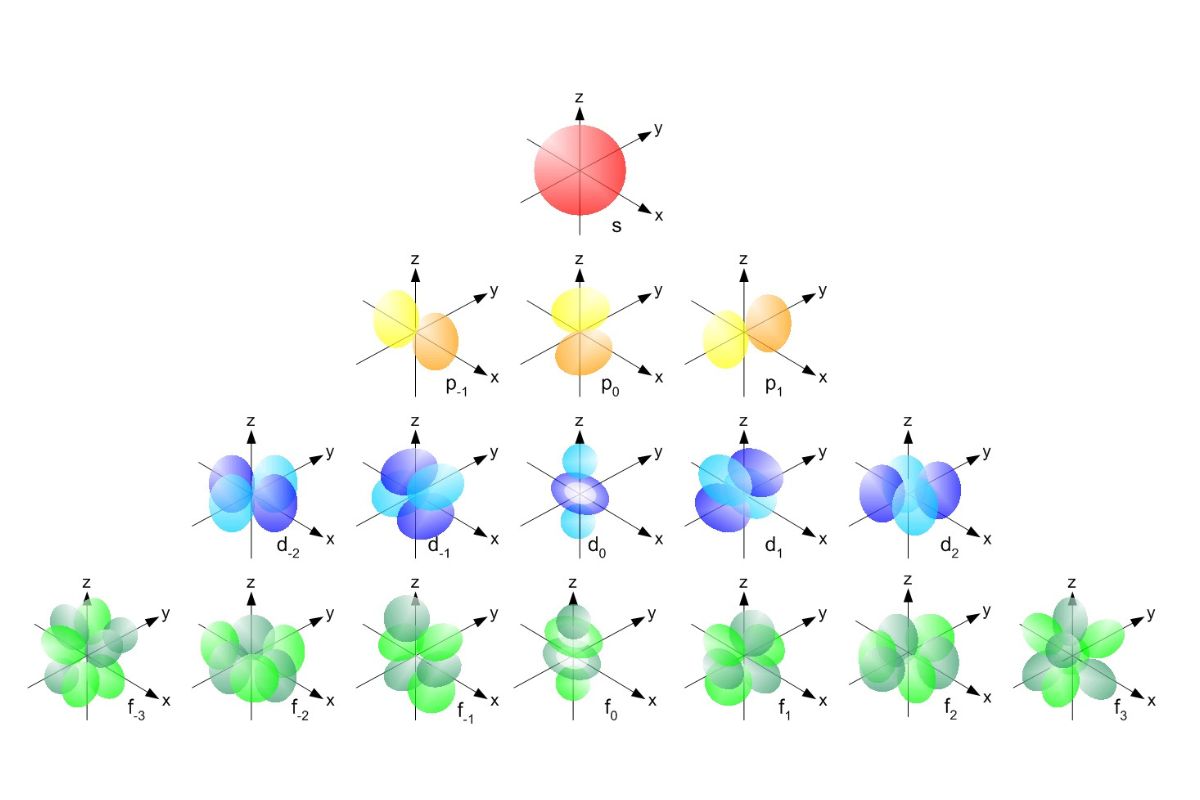
- Shape: s orbitals are spherical in shape, meaning they are symmetrical around the nucleus.
- Energy Levels: Each energy level has one s orbital. For example, the first energy level has a 1s orbital, the second has a 2s orbital, and so on.
- Electron Capacity: Each s orbital can hold a maximum of two electrons.
- Nodal Surfaces: Higher energy s orbitals (like 2s, 3s) have spherical nodes where the probability of finding an electron is zero.
- Penetration: s orbitals have the highest penetration ability, meaning they can get closer to the nucleus compared to p, d, and f orbitals.
p Orbitals
p orbitals are more complex than s orbitals and have distinct shapes and orientations.
- Shape: p orbitals are dumbbell-shaped and have two lobes on opposite sides of the nucleus.
- Orientation: There are three p orbitals (px, py, pz) at each energy level, oriented along the x, y, and z axes.
- Energy Levels: p orbitals start appearing at the second energy level (2p).
- Electron Capacity: Each p orbital can hold two electrons, so a set of three p orbitals can hold a total of six electrons.
- Nodal Planes: p orbitals have a nodal plane, a region where the probability of finding an electron is zero, passing through the nucleus.
d Orbitals
d orbitals are even more complex and have unique shapes and properties.
- Shape: d orbitals have more complex shapes, including cloverleaf patterns and a doughnut-shaped ring.
- Orientation: There are five d orbitals (dxy, dyz, dxz, dx2-y2, dz2) with different orientations in space.
- Energy Levels: d orbitals start appearing at the third energy level (3d).
- Electron Capacity: Each d orbital can hold two electrons, so a set of five d orbitals can hold a total of ten electrons.
- Nodal Surfaces: d orbitals have more complex nodal surfaces compared to s and p orbitals.
f Orbitals
f orbitals are the most complex and have intricate shapes and properties.
- Shape: f orbitals have very complex shapes, often described as having multiple lobes and intricate patterns.
- Orientation: There are seven f orbitals with different orientations in space.
- Energy Levels: f orbitals start appearing at the fourth energy level (4f).
- Electron Capacity: Each f orbital can hold two electrons, so a set of seven f orbitals can hold a total of fourteen electrons.
- Nodal Surfaces: f orbitals have the most complex nodal surfaces of all the orbitals.
General Facts About Orbitals
Orbitals, in general, have some fascinating properties that apply to all types.
- Quantum Numbers: Each orbital is defined by a set of quantum numbers (n, l, m), which describe its size, shape, and orientation.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers, meaning each orbital can hold a maximum of two electrons with opposite spins.
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level to the highest.
- Hund's Rule: Electrons will fill degenerate orbitals (orbitals with the same energy) singly before pairing up.
- Electron Configuration: The arrangement of electrons in an atom's orbitals is called its electron configuration, which determines the atom's chemical properties.
Interesting Applications
Orbitals play a crucial role in various scientific fields and applications.
- Chemical Bonding: The shape and orientation of orbitals determine how atoms bond with each other to form molecules.
- Spectroscopy: The study of how atoms absorb and emit light involves understanding electron transitions between orbitals.
- Quantum Chemistry: Orbitals are fundamental to quantum chemistry, which explains the behavior of electrons in atoms and molecules.
- Magnetism: The arrangement of electrons in orbitals can explain the magnetic properties of materials.
- Periodic Table: The structure of the periodic table is based on the filling order of atomic orbitals.
Fun Facts
Orbitals also have some fun and quirky aspects that make them even more interesting.
- Electron Clouds: Orbitals are often visualized as electron clouds, where the density of the cloud represents the probability of finding an electron.
- Hybrid Orbitals: In molecules, atomic orbitals can mix to form hybrid orbitals, which have different shapes and properties.
- Molecular Orbitals: When atoms bond, their atomic orbitals combine to form molecular orbitals, which belong to the entire molecule.
- Orbital Overlap: The strength of a chemical bond depends on the extent of overlap between the orbitals of the bonding atoms.
- Excited States: When atoms absorb energy, electrons can jump to higher orbitals, creating excited states.
- Electron Spin: Electrons have a property called spin, which can be either up or down, and this affects how they fill orbitals.
- Visualization Tools: Modern software allows scientists to visualize and study the shapes and properties of orbitals in great detail.
The Final Word on Orbitals
Understanding s, p, d, and f orbitals is crucial for grasping the basics of chemistry. These orbitals define how electrons are arranged around an atom's nucleus, influencing everything from chemical reactions to the properties of elements. The s orbital is spherical, the p orbital is dumbbell-shaped, while d and f orbitals have more complex shapes. Each type of orbital can hold a specific number of electrons, which determines the element's behavior in reactions. Knowing these facts helps in predicting how elements will interact, making it easier to understand the periodic table and chemical bonding. Whether you're a student or just curious about science, these insights into orbitals provide a solid foundation for further exploration. Keep these facts in mind, and you'll have a better grasp of the microscopic world that shapes our universe.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
