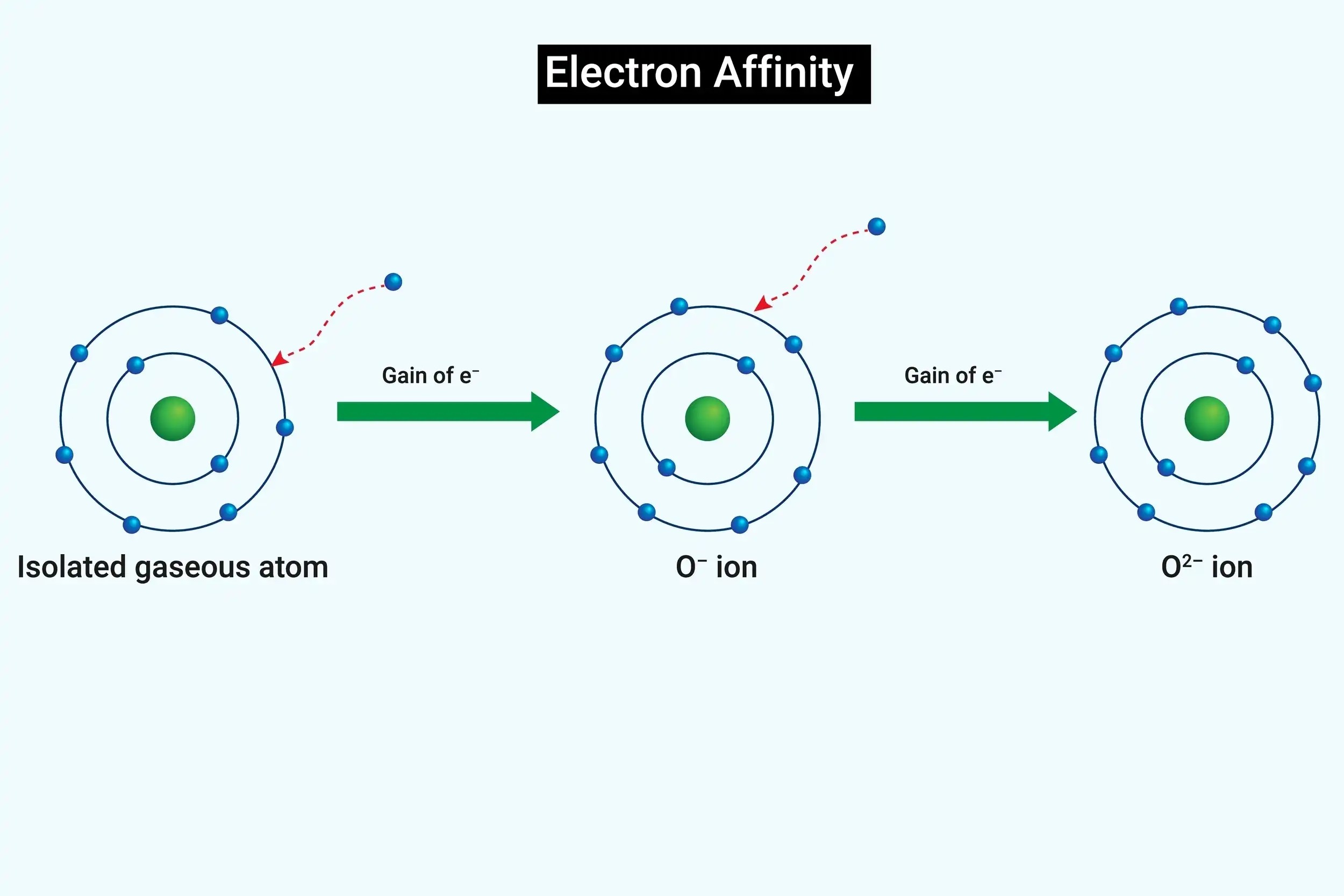
What is electron affinity? Electron affinity measures how much energy is released when an atom gains an electron. It’s like the atom's eagerness to grab an extra electron. Elements with high electron affinity, like chlorine, release a lot of energy when they gain an electron. This property helps explain why some elements form certain compounds. For example, in table salt, sodium gives up an electron while chlorine eagerly accepts it. Understanding electron affinity can help predict chemical reactions and bonding behavior. Ready to dive into 36 fascinating facts about electron affinity? Let’s get started!
What is Electron Affinity?
Electron affinity measures how much energy is released when an atom gains an electron. This concept is crucial in chemistry, helping to understand how atoms interact and form bonds. Let's dive into some fascinating facts about electron affinity.
-
Electron affinity is measured in kilojoules per mole (kJ/mol). This unit quantifies the energy change when one mole of atoms gains electrons.
-
Halogens have the highest electron affinities. Elements like fluorine and chlorine release a lot of energy when gaining an electron, making them highly reactive.
-
Noble gases have low electron affinities. These elements, like helium and neon, are stable and don't easily gain electrons.
-
Electron affinity can be positive or negative. A negative value means energy is released, while a positive value indicates energy is required.
-
Fluorine has the highest electron affinity. Among all elements, fluorine releases the most energy when it gains an electron.
Factors Affecting Electron Affinity
Several factors influence an element's electron affinity. Understanding these can help predict how different elements will behave in chemical reactions.
-
Atomic size affects electron affinity. Smaller atoms have higher electron affinities because the added electron is closer to the nucleus.
-
Nuclear charge impacts electron affinity. A higher positive charge in the nucleus attracts electrons more strongly, increasing electron affinity.
-
Electron shielding reduces electron affinity. Inner electrons can shield outer electrons from the nucleus's pull, lowering electron affinity.
-
Electron configuration plays a role. Atoms with nearly full or empty outer shells have higher electron affinities.
-
Periodicity influences electron affinity. Elements in the same group or period often show similar electron affinity trends.
Trends in the Periodic Table
Electron affinity shows clear trends across the periodic table. These trends help predict how elements will interact.
-
Electron affinity increases across a period. Moving from left to right, atoms gain more protons, increasing their ability to attract electrons.
-
Electron affinity decreases down a group. As atoms get larger, the added electron is farther from the nucleus, reducing electron affinity.
-
Transition metals have variable electron affinities. These elements can have complex electron configurations, leading to varied affinities.
-
Lanthanides and actinides show irregular trends. These elements have unique electron configurations, making their electron affinities less predictable.
-
Metalloids have intermediate electron affinities. These elements, like silicon and arsenic, fall between metals and nonmetals in their ability to attract electrons.
Applications of Electron Affinity
Understanding electron affinity has practical applications in various fields, from chemistry to materials science.
-
Predicting chemical reactivity. Elements with high electron affinities are more likely to gain electrons and form negative ions.
-
Designing new materials. Knowledge of electron affinity helps in creating materials with specific electronic properties.
-
Developing batteries. Electron affinity plays a role in the efficiency and capacity of battery materials.
-
Understanding catalysis. Catalysts often rely on elements with specific electron affinities to speed up reactions.
-
Exploring semiconductor properties. Electron affinity is crucial in designing semiconductors for electronics.
Interesting Facts About Specific Elements
Some elements have particularly notable electron affinities, making them stand out in the periodic table.
-
Chlorine has a high electron affinity. This element is highly reactive and commonly forms negative ions.
-
Oxygen's electron affinity is crucial for life. Oxygen's ability to gain electrons is essential for processes like respiration.
-
Sulfur has a moderate electron affinity. This element's affinity influences its role in biological systems and industrial processes.
-
Nitrogen has a low electron affinity. Despite being essential for life, nitrogen doesn't easily gain electrons.
-
Carbon's electron affinity affects organic chemistry. Carbon's ability to form stable bonds with other elements is key to organic compounds.
Historical Context and Discoveries
The concept of electron affinity has evolved over time, with significant contributions from various scientists.
-
Electron affinity was first measured in the early 20th century. Advances in spectroscopy allowed scientists to quantify this property.
-
Linus Pauling contributed to understanding electron affinity. His work on electronegativity and bonding helped clarify how electron affinity influences chemical behavior.
-
Quantum mechanics explains electron affinity. The development of quantum theory provided a deeper understanding of how electrons interact with atoms.
-
Electron affinity research continues today. Scientists are still exploring how this property affects new materials and technologies.
-
Electron affinity is linked to electronegativity. Both properties describe an element's ability to attract electrons, though they are measured differently.
Fun Facts and Trivia
Let's wrap up with some fun and lesser-known facts about electron affinity.
-
Electron affinity can be counterintuitive. Some elements, like noble gases, have unexpected electron affinities due to their stable configurations.
-
Electron affinity varies with temperature. Higher temperatures can affect how easily atoms gain electrons.
-
Electron affinity influences color. The way elements interact with light can be affected by their electron affinities.
-
Electron affinity is important in astrophysics. Understanding how elements gain electrons helps explain stellar and planetary formation.
-
Electron affinity affects corrosion. Elements with high electron affinities can influence how materials corrode over time.
-
Electron affinity is used in spectroscopy. This property helps identify elements based on their spectral lines.
The Final Word on Electron Affinity
Electron affinity is a fascinating topic in chemistry. It measures how much energy is released when an atom gains an electron. Elements like chlorine and fluorine have high electron affinities, making them highly reactive. This property helps explain why some elements form certain compounds and not others. Understanding electron affinity can also shed light on trends in the periodic table, such as why elements in the same group behave similarly.
Knowing these facts can help students, scientists, and anyone curious about chemistry grasp how atoms interact. It’s a key concept that plays a role in everything from the formation of molecules to the behavior of materials. So next time you think about chemical reactions, remember the role electron affinity plays. It’s a small but mighty force in the world of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
