What is the Quantum Mechanical Model? The Quantum Mechanical Model is a fundamental theory in physics that describes the behavior of particles at the atomic and subatomic levels. Unlike classical physics, which treats particles as solid objects, this model views them as wave functions, providing probabilities of where particles might be found. This approach helps explain phenomena that classical physics can't, such as the behavior of electrons in atoms. Developed in the early 20th century by scientists like Schrödinger and Heisenberg, it revolutionized our understanding of the microscopic world. Quantum mechanics is crucial for technologies like semiconductors, lasers, and even MRI machines. Understanding this model opens up a world where particles can exist in multiple states at once, leading to mind-bending concepts like superposition and entanglement.
What is the Quantum Mechanical Model?
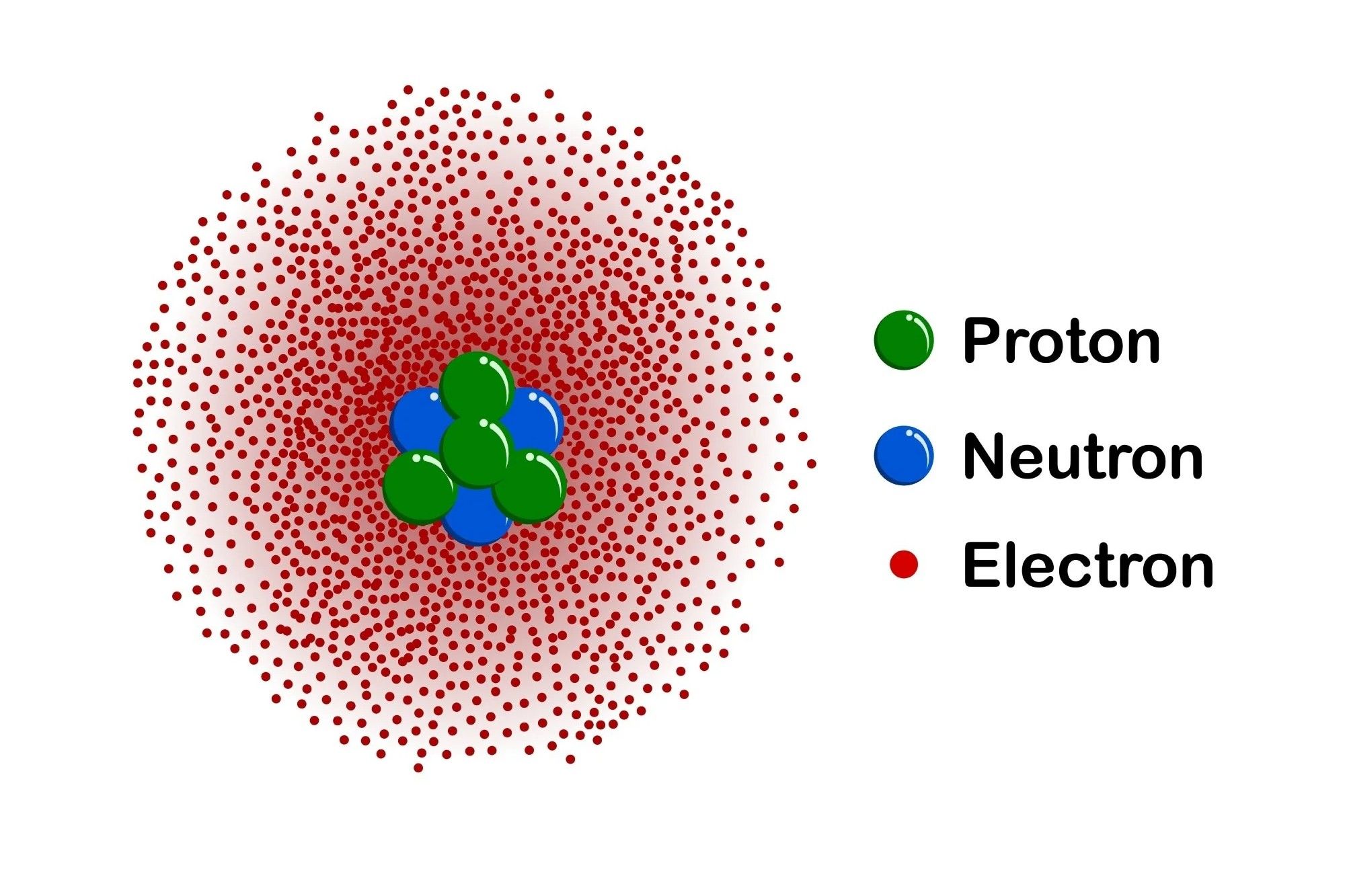
The quantum mechanical model of the atom is a fundamental theory in physics that describes the behavior of electrons in atoms. Unlike classical models, it incorporates principles of quantum mechanics to explain atomic structure and electron behavior.
-
Wave-Particle Duality: Electrons exhibit both wave-like and particle-like properties. This dual nature is a cornerstone of quantum mechanics.
-
Heisenberg Uncertainty Principle: It's impossible to know both the exact position and momentum of an electron simultaneously. This principle introduces a fundamental limit to what can be known about atomic particles.
-
Schrödinger Equation: This mathematical equation describes how the quantum state of a physical system changes over time. It’s central to the quantum mechanical model.
-
Electron Clouds: Instead of fixed orbits, electrons exist in probabilistic clouds around the nucleus. These clouds represent areas where electrons are likely to be found.
-
Quantum Numbers: Four quantum numbers describe the properties of electrons: principal (n), angular momentum (l), magnetic (m), and spin (s). Each number provides specific information about an electron's energy and position.
Historical Development of the Quantum Mechanical Model
The development of the quantum mechanical model was a collaborative effort by several pioneering scientists. Their contributions laid the groundwork for modern quantum mechanics.
-
Max Planck: Introduced the idea of quantized energy levels, which was a significant departure from classical physics.
-
Albert Einstein: Explained the photoelectric effect using quantum theory, showing that light can be thought of as particles called photons.
-
Niels Bohr: Proposed the Bohr model of the atom, which introduced quantized electron orbits but was later refined by quantum mechanics.
-
Erwin Schrödinger: Developed the Schrödinger equation, which describes how the quantum state of a system evolves.
-
Werner Heisenberg: Formulated the uncertainty principle and matrix mechanics, another formulation of quantum mechanics.
Key Concepts in Quantum Mechanics
Several key concepts are essential for understanding the quantum mechanical model. These ideas challenge classical notions and provide a new framework for atomic theory.
-
Superposition: An electron can exist in multiple states at once until it is observed. This principle is famously illustrated by Schrödinger's cat thought experiment.
-
Entanglement: Particles can become entangled, meaning the state of one particle instantly influences the state of another, no matter the distance between them.
-
Quantum Tunneling: Electrons can pass through energy barriers that they classically shouldn't be able to. This phenomenon is crucial in many modern technologies.
-
Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers. This principle explains the structure of the periodic table.
-
Wave Function: Describes the quantum state of a particle and contains all the information about the system. The square of the wave function gives the probability density of finding a particle in a particular location.
Applications of the Quantum Mechanical Model
The principles of quantum mechanics have led to numerous technological advancements and practical applications.
-
Semiconductors: Quantum mechanics explains the behavior of electrons in semiconductors, which are the foundation of modern electronics.
-
Lasers: Operate based on the principles of quantum mechanics, specifically the stimulated emission of photons.
-
MRI Machines: Use quantum mechanics to produce detailed images of the inside of the human body.
-
Quantum Computing: Utilizes the principles of superposition and entanglement to perform computations much faster than classical computers.
-
Cryptography: Quantum mechanics offers new methods for secure communication, such as quantum key distribution.
Misconceptions About Quantum Mechanics
Despite its success, quantum mechanics is often misunderstood. Here are some common misconceptions.
-
Determinism: Unlike classical physics, quantum mechanics is inherently probabilistic, not deterministic.
-
Observer Effect: Observation in quantum mechanics doesn't necessarily mean a conscious observer; it can be any interaction that causes a quantum system to collapse into a definite state.
-
Size Limitation: Quantum effects are not limited to microscopic particles; they can also affect macroscopic systems under certain conditions.
-
Instantaneous Action: Entanglement doesn't allow for faster-than-light communication; it merely shows correlations between entangled particles.
-
Wave-Function Collapse: The collapse of the wave function is not a physical process but a change in our knowledge about the system.
Quantum Mechanics in Popular Culture
Quantum mechanics has captured the imagination of many and has been featured in various forms of popular culture.
-
Science Fiction: Movies like "Interstellar" and "Ant-Man" explore concepts like time dilation and quantum realms.
-
Literature: Books such as "The Quantum Thief" incorporate quantum mechanics into their plots.
-
Television: Shows like "The Big Bang Theory" often reference quantum mechanics in a humorous context.
-
Video Games: Games like "Quantum Break" use quantum mechanics as a central theme in their storylines.
-
Music: Some musicians have been inspired by quantum mechanics, creating albums and songs that reference its concepts.
Future of Quantum Mechanics
Quantum mechanics continues to be a vibrant field of research with many exciting possibilities on the horizon.
-
Quantum Internet: Researchers are working on developing a quantum internet, which would allow for ultra-secure communication.
-
Quantum Sensors: These devices could revolutionize fields like medicine and environmental monitoring by providing unprecedented sensitivity.
-
Quantum Materials: New materials with unique properties, such as superconductors, are being developed based on quantum principles.
-
Quantum Biology: Scientists are exploring the role of quantum mechanics in biological processes, such as photosynthesis and bird navigation.
-
Quantum Gravity: Efforts are ongoing to develop a theory of quantum gravity, which would unify quantum mechanics with general relativity.
The Quantum World in a Nutshell
Quantum mechanics is mind-blowing. From wave-particle duality to quantum entanglement, it challenges our understanding of reality. The Heisenberg Uncertainty Principle shows that we can't know everything about a particle at once. Schrödinger's cat illustrates the weirdness of quantum superposition. Quantum tunneling lets particles pass through barriers, defying classical physics.
These concepts aren't just theoretical. They power technologies like MRI machines, lasers, and quantum computers. Quantum mechanics is reshaping fields from cryptography to telecommunications.
Understanding these facts can help us appreciate the universe's complexity. Quantum mechanics isn't just for scientists; it's for anyone curious about how the world works. Keep exploring, keep questioning, and who knows? You might just stumble upon the next big breakthrough in quantum theory.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
