Allotropes are different forms of the same element, each with distinct physical and chemical properties. Carbon, for instance, can exist as diamond, graphite, or graphene. These variations occur because atoms in allotropes bond differently. Oxygen has two common allotropes: O2 (the oxygen we breathe) and O3 (ozone). Phosphorus can be red, white, or black, each with unique traits. Understanding allotropes helps us grasp why materials behave differently. For example, diamonds are incredibly hard due to their atomic structure, while graphite is soft and slippery. This knowledge is crucial in fields like chemistry, physics, and materials science. Ready to learn more? Let's dive into 35 fascinating facts about allotropes!
What Are Allotropes?
Allotropes are different forms of the same element, where atoms are bonded together in different ways. This leads to materials with distinct physical and chemical properties. Let's dive into some fascinating facts about allotropes.
-
Carbon's Versatility
Carbon has several allotropes, including diamond, graphite, and graphene. Each has unique properties due to different atomic arrangements. -
Diamond's Hardness
Diamond, an allotrope of carbon, is the hardest natural material known. Its atoms form a rigid three-dimensional lattice. -
Graphite's Lubrication
Graphite, another carbon allotrope, is soft and slippery. Its layers can slide over each other, making it an excellent lubricant. -
Graphene's Strength
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, is incredibly strong and conductive. It's 200 times stronger than steel. -
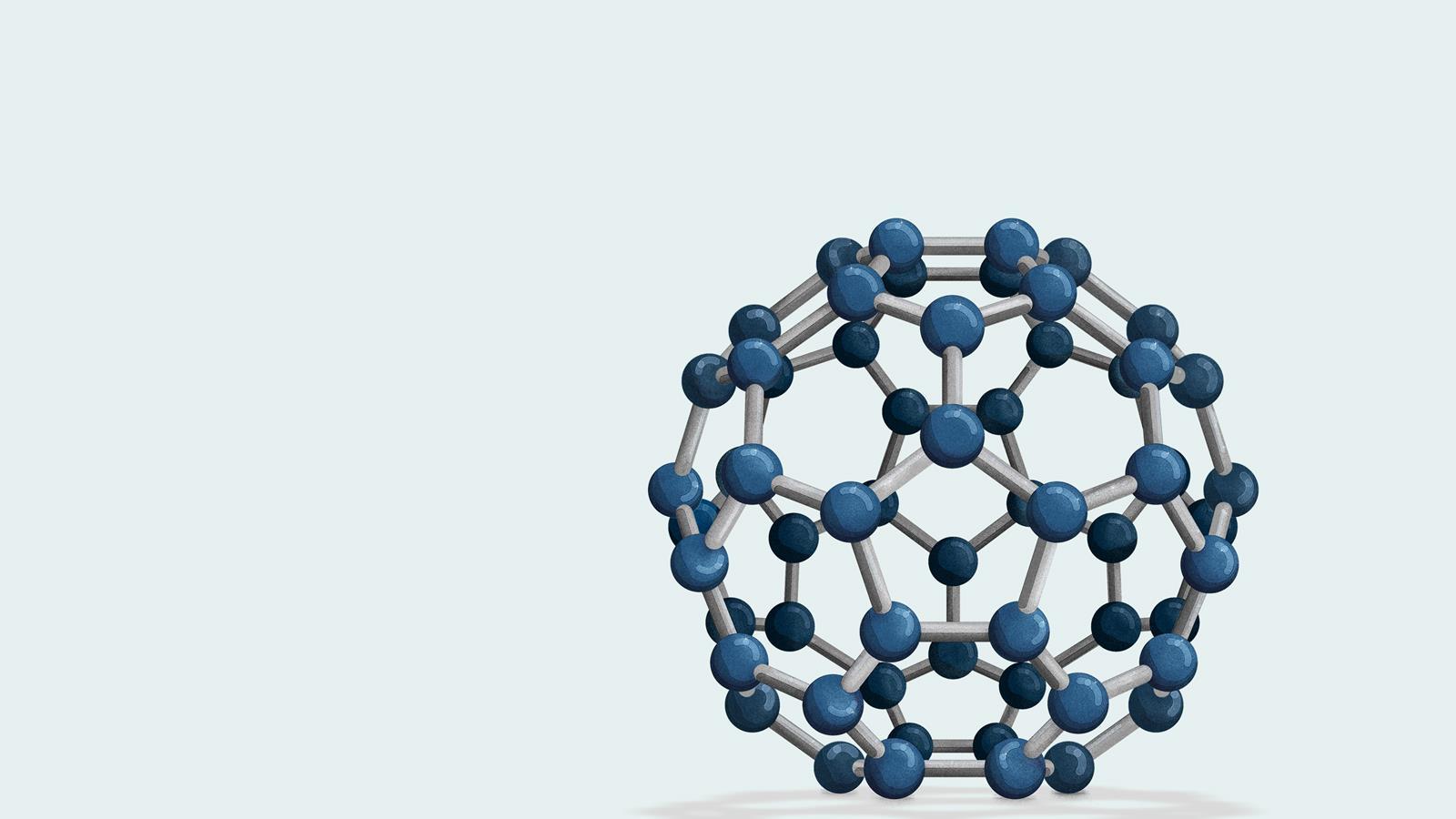
Fullerenes' Discovery
Fullerenes, also known as buckyballs, are spherical carbon allotropes discovered in 1985. They resemble a soccer ball in structure.
Allotropes of Oxygen
Oxygen, essential for life, also has different allotropes with unique properties. Let's explore these forms.
-
Diatomic Oxygen (O2)
The most common form of oxygen is diatomic oxygen (O2), which we breathe. It's essential for respiration in most living organisms. -
Ozone (O3)
Ozone (O3) is a triatomic molecule found in the Earth's stratosphere. It protects us from harmful ultraviolet radiation. -
Ozone's Smell
Ozone has a distinct, sharp smell often noticed after thunderstorms. It's produced by lightning splitting O2 molecules, which then recombine to form O3.
Sulfur's Allotropes
Sulfur, known for its yellow color and distinct smell, has several allotropes with varying properties.
-
Rhombic Sulfur
Rhombic sulfur is the most stable allotrope at room temperature. It forms yellow crystals with a specific orthorhombic structure. -
Monoclinic Sulfur
Monoclinic sulfur forms needle-like crystals and is stable at higher temperatures. It reverts to rhombic sulfur upon cooling. -
Plastic Sulfur
Plastic sulfur is an amorphous form created by quickly cooling molten sulfur. It's rubbery and flexible but eventually crystallizes into rhombic sulfur.
Phosphorus Allotropes
Phosphorus, essential for life, exists in several allotropes with distinct characteristics.
-
White Phosphorus
White phosphorus is highly reactive and glows in the dark. It's stored underwater to prevent it from reacting with oxygen. -
Red Phosphorus
Red phosphorus is more stable and less reactive than white phosphorus. It's used in safety matches and fireworks. -
Black Phosphorus
Black phosphorus has a layered structure similar to graphite. It's a good conductor of electricity and has potential in electronics.
Selenium's Forms
Selenium, a trace element important for health, has several allotropes with unique properties.
-
Amorphous Selenium
Amorphous selenium is a glass-like form used in photocopiers and solar cells due to its photoconductive properties. -
Crystalline Selenium
Crystalline selenium exists in two forms: trigonal and monoclinic. Trigonal selenium is the most stable and conductive.
Tin's Allotropes
Tin, a common metal, has two main allotropes with different properties.
-
White Tin
White tin is the metallic form stable at room temperature. It's malleable and used in coatings and alloys. -
Gray Tin
Gray tin is a brittle, non-metallic form stable at low temperatures. It can cause "tin pest," where white tin transforms into gray tin, leading to disintegration.
Arsenic's Allotropes
Arsenic, known for its toxicity, has several allotropes with distinct characteristics.
-
Gray Arsenic
Gray arsenic is the most stable and metallic form. It's used in semiconductors and alloys. -
Yellow Arsenic
Yellow arsenic is a molecular form that is highly unstable and toxic. It quickly transforms into gray arsenic.
Silicon's Allotropes
Silicon, essential in electronics, has several allotropes with unique properties.
-
Crystalline Silicon
Crystalline silicon is the most common form used in semiconductors and solar cells. It has a diamond-like structure. -
Amorphous Silicon
Amorphous silicon is a non-crystalline form used in thin-film solar cells and LCDs. It's less efficient but cheaper to produce.
Boron's Allotropes
Boron, a metalloid, has several allotropes with varying properties.
-
Alpha Boron
Alpha boron is a hard, black, and brittle form with a rhombohedral structure. It's used in high-strength materials. -
Beta Boron
Beta boron is more stable than alpha boron and has a similar structure. It's used in boron fibers and ceramics.
Iron's Allotropes
Iron, a common metal, has several allotropes with different properties.
-
Alpha Iron
Alpha iron, or ferrite, is stable at room temperature. It's magnetic and used in steel production. -
Gamma Iron
Gamma iron, or austenite, is stable at high temperatures. It's non-magnetic and used in stainless steel.
Allotropes of Antimony
Antimony, a metalloid, has several allotropes with distinct characteristics.
-
Metallic Antimony
Metallic antimony is the most stable form, used in alloys and flame retardants. -
Yellow Antimony
Yellow antimony is a molecular form that is highly unstable and quickly transforms into metallic antimony.
Allotropes of Tellurium
Tellurium, a rare metalloid, has several allotropes with unique properties.
-
Crystalline Tellurium
Crystalline tellurium is the most stable form, used in alloys and semiconductors. -
Amorphous Tellurium
Amorphous tellurium is a non-crystalline form used in thin-film solar cells and thermoelectric devices.
Allotropes of Polonium
Polonium, a rare and highly radioactive element, has several allotropes with distinct characteristics.
-
Alpha Polonium
Alpha polonium is the most stable form, used in anti-static devices and neutron sources. -
Beta Polonium
Beta polonium is a less stable form that quickly transforms into alpha polonium.
Allotropes of Bismuth
Bismuth, a brittle metal, has several allotropes with unique properties.
-
Metallic Bismuth
Metallic bismuth is the most stable form, used in alloys and pharmaceuticals. -
Amorphous Bismuth
Amorphous bismuth is a non-crystalline form with potential applications in electronics and materials science.
Allotropes of Plutonium
Plutonium, a radioactive element, has several allotropes with distinct characteristics.
- Alpha Plutonium
Alpha plutonium is the most stable form at room temperature, used in nuclear reactors and weapons.
The Fascinating World of Allotropes
Allotropes show how versatile elements can be. From diamonds to graphite, carbon alone has some amazing forms. Oxygen can be both life-saving and explosive, depending on its allotrope. Phosphorus has a dark side and a glowing side. These variations aren't just cool science facts; they have real-world applications. For instance, graphene is revolutionizing electronics, while fullerenes are making strides in medicine. Understanding allotropes helps us appreciate the complexity and beauty of the elements around us. Next time you use a pencil or take a breath, remember the incredible science behind these everyday materials. Keep exploring, and you'll find even more wonders in the world of allotropes.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
