Stereoisomers are fascinating molecules that share the same molecular formula but differ in the spatial arrangement of their atoms. This unique characteristic can lead to vastly different properties and behaviors in chemical reactions. Why should you care about stereoisomers? Because they play crucial roles in fields like pharmaceuticals, agriculture, and even food science. Imagine two compounds with identical chemical compositions, yet one is a life-saving drug while the other is ineffective or even harmful. Understanding stereoisomers can help us grasp why this happens. In this post, we'll explore 32 intriguing facts about stereoisomers, shedding light on their importance and the science behind them. Buckle up for a journey through the world of molecular twists and turns!
What Are Stereoisomers?
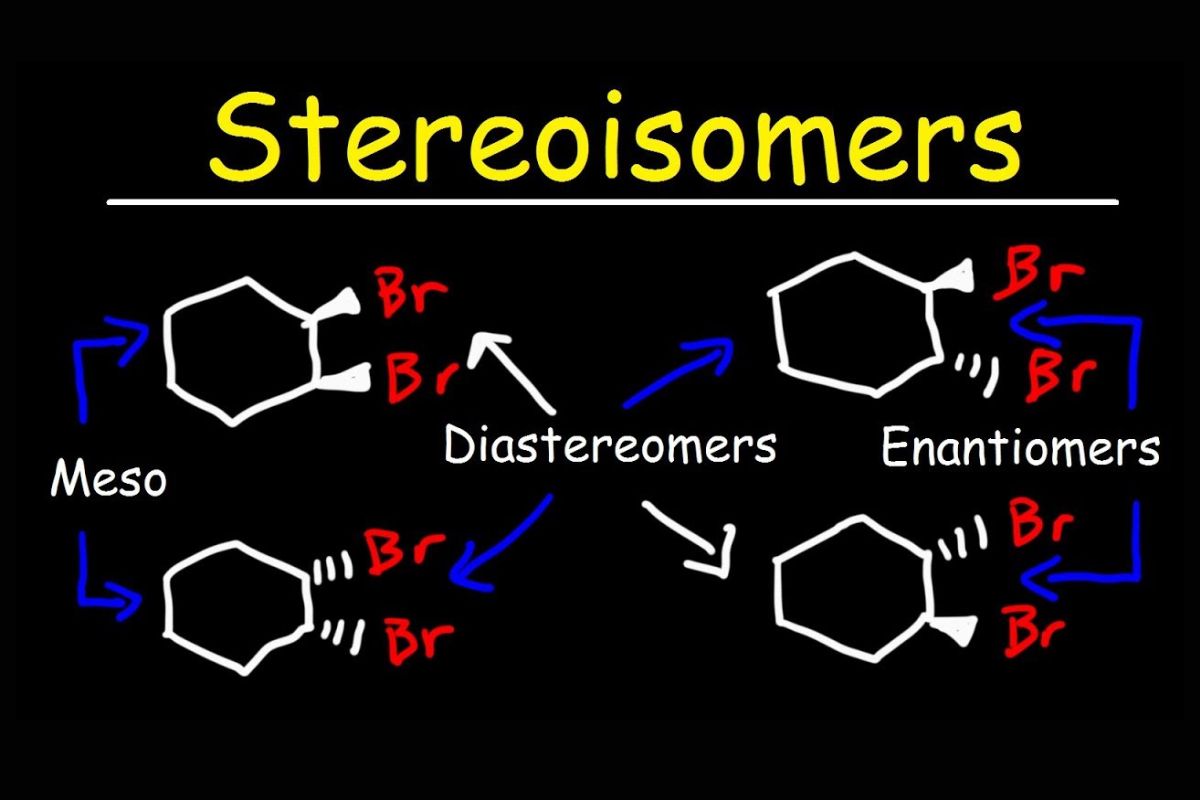
Stereoisomers are fascinating molecules that have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms. This difference can lead to unique properties and behaviors. Let's dive into some intriguing facts about stereoisomers.
-
Stereoisomers include enantiomers and diastereomers. Enantiomers are mirror images of each other, while diastereomers are not.
-
Enantiomers have identical physical properties. They share the same melting point, boiling point, and density but differ in the way they rotate plane-polarized light.
-
Chirality is key to stereoisomers. A molecule is chiral if it cannot be superimposed on its mirror image, much like left and right hands.
-
Louis Pasteur discovered enantiomers. In 1848, he separated tartaric acid crystals into two types, discovering their optical activity.
-
Diastereomers have different physical properties. Unlike enantiomers, diastereomers can have different melting points, solubilities, and densities.
Importance in Pharmaceuticals
Stereoisomers play a crucial role in the pharmaceutical industry. The effectiveness and safety of drugs can depend on their stereochemistry.
-
Thalidomide tragedy highlighted stereoisomer importance. One enantiomer of thalidomide caused birth defects, while the other was effective as a sedative.
-
Ibuprofen is sold as a racemic mixture. This means it contains both enantiomers, but only one is active in relieving pain.
-
Stereoisomers can have different biological activities. One enantiomer might be beneficial, while the other could be harmful or inactive.
-
Chiral drugs are often more effective. They can target specific biological pathways more precisely, reducing side effects.
-
Regulations require testing of each stereoisomer. Drug approval processes now mandate the study of each enantiomer's effects.
Stereoisomers in Nature
Nature is full of stereoisomers, influencing everything from taste to smell to biological functions.
-
Carvone enantiomers smell different. One enantiomer smells like spearmint, while the other smells like caraway.
-
Amino acids are chiral. Most naturally occurring amino acids are in the L-form, essential for protein synthesis.
-
Sugars have stereoisomers. Glucose and galactose are diastereomers, differing only in the orientation of one hydroxyl group.
-
Limonene enantiomers have distinct scents. One smells like oranges, the other like lemons.
-
Plants produce specific stereoisomers. Many plant-derived compounds, like menthol, exist as specific enantiomers.
Stereoisomers in Food and Flavor
The food industry leverages stereoisomers to enhance flavors and aromas, making our culinary experiences more enjoyable.
-
Aspartame has stereoisomers. Only one form is sweet, used as a low-calorie sweetener.
-
Vanillin exists as stereoisomers. Natural vanilla flavor comes from one specific enantiomer.
-
Stereoisomers affect wine aroma. Different enantiomers of compounds like linalool contribute to the complex bouquet of wines.
-
Coriander and cilantro taste difference. The same plant produces different flavors due to stereoisomers in its essential oils.
-
Stereoisomers in spices. Compounds like cinnamaldehyde have different flavors depending on their stereochemistry.
Stereoisomers in Technology and Industry
Beyond biology and food, stereoisomers have applications in technology and industry, influencing materials and processes.
-
Liquid crystals use stereoisomers. The orientation of molecules affects the display properties in screens.
-
Polymers can be stereoisomers. Isotactic, syndiotactic, and atactic polymers have different properties based on their stereochemistry.
-
Stereoisomers in agrochemicals. Herbicides and pesticides often rely on specific enantiomers for effectiveness.
-
Catalysts can be chiral. Chiral catalysts are used to produce specific stereoisomers in chemical reactions.
-
Stereoisomers in fragrances. The perfume industry uses specific enantiomers to create desired scents.
Fun and Quirky Facts
Stereoisomers can also be fun and quirky, influencing everyday experiences in unexpected ways.
-
Handedness in snails. Some snail species have shells that spiral in opposite directions due to stereoisomerism.
-
Stereoisomers in space. Certain amino acids found in meteorites are predominantly one enantiomer, hinting at the origins of life.
-
Stereoisomers in art. Some artists use chiral molecules to create unique visual effects in their work.
-
Stereoisomers in music. The shape of musical instruments can affect sound, similar to how stereochemistry affects molecular properties.
-
Stereoisomers in sports. The design of sports equipment, like golf clubs, can be influenced by stereochemistry for better performance.
-
Stereoisomers in fashion. The twist of fibers in fabrics can affect texture and appearance, much like molecular chirality.
-
Stereoisomers in everyday products. From cleaning agents to cosmetics, stereochemistry plays a role in the effectiveness and safety of many products we use daily.
The Fascinating World of Stereoisomers
Stereoisomers are more than just a chemistry concept. They play a crucial role in everyday life, from the medicines we take to the food we eat. Understanding enantiomers and diastereomers can help us appreciate the complexity and beauty of molecular structures. These molecules can have vastly different effects despite having the same chemical formula, highlighting the importance of chirality in pharmaceuticals and other industries.
Knowing about stereoisomers can also shed light on why certain drugs work better than others or why some foods taste different. It's a reminder of how intricate and interconnected our world is. So next time you hear about a new drug or taste a unique flavor, remember that stereoisomers might be behind it. Keep exploring and stay curious—there's always more to learn about the tiny building blocks that shape our world.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
