Vapor pressure might sound like a complex term, but it's actually quite simple. Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid form. This concept is crucial in understanding how liquids evaporate and how they behave under different temperatures. For instance, when you boil water, the steam you see is water vapor escaping due to increased vapor pressure. This pressure varies with temperature; higher temperatures mean higher vapor pressure. Knowing about vapor pressure helps in fields like meteorology, cooking, and even in understanding how our bodies sweat. Ready to dive into some fascinating facts about vapor pressure? Let's get started!
What is Vapor Pressure?
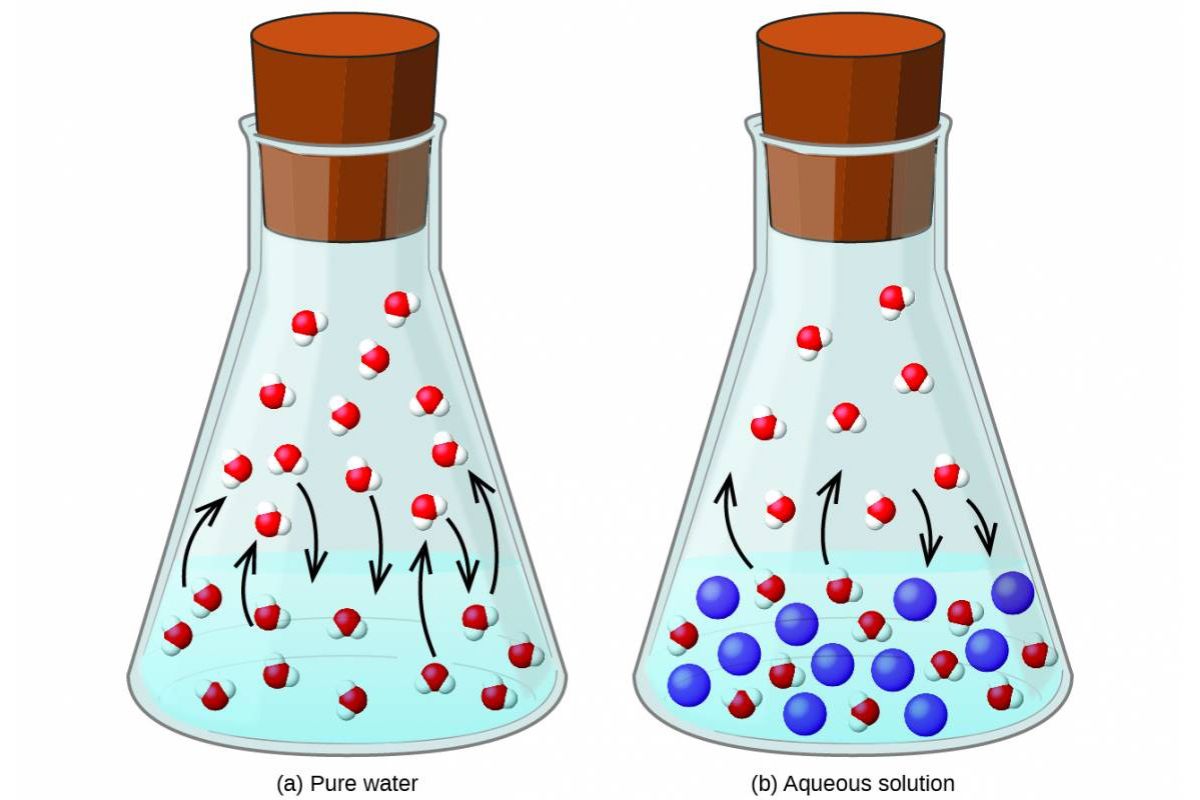
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase. Understanding vapor pressure helps explain why liquids evaporate and how they behave under different conditions.
- Vapor pressure is a measure of a liquid's tendency to evaporate. Higher vapor pressure means the liquid evaporates more easily.
- Temperature directly affects vapor pressure. As temperature increases, vapor pressure rises.
- Boiling point occurs when vapor pressure equals atmospheric pressure. Water boils at 100°C (212°F) at sea level.
- Volatile substances have high vapor pressures. Examples include alcohol and gasoline.
- Non-volatile substances like oil have low vapor pressures and evaporate slowly.
- Dynamic equilibrium is reached when the rate of evaporation equals the rate of condensation in a closed system.
- Saturation vapor pressure is the maximum pressure exerted by a vapor in equilibrium with its liquid at a given temperature.
- Humidity is related to vapor pressure. Relative humidity is the ratio of the current vapor pressure to the saturation vapor pressure.
- Raoult's Law states that the vapor pressure of a solution is directly proportional to the mole fraction of the solvent.
- Partial pressure is the pressure exerted by a single component of a mixture of gases.
Factors Affecting Vapor Pressure
Several factors influence vapor pressure, including the nature of the liquid and external conditions. Understanding these factors helps predict how substances will behave in different environments.
- Intermolecular forces affect vapor pressure. Stronger forces result in lower vapor pressure.
- Molecular weight influences vapor pressure. Heavier molecules generally have lower vapor pressures.
- Surface area impacts evaporation rate but not vapor pressure. Larger surface areas allow more molecules to escape.
- Impurities can alter vapor pressure. Adding a non-volatile solute lowers the vapor pressure of a solvent.
- Altitude affects boiling points but not vapor pressure directly. Higher altitudes have lower atmospheric pressure, lowering boiling points.
Applications of Vapor Pressure
Vapor pressure plays a crucial role in various scientific and industrial applications. It helps in designing processes and understanding natural phenomena.
- Distillation relies on differences in vapor pressure to separate components of a mixture.
- Refrigeration uses substances with specific vapor pressures to absorb and release heat efficiently.
- Meteorology uses vapor pressure to predict weather patterns and humidity levels.
- Perfume industry selects ingredients based on their vapor pressures to control evaporation rates.
- Food preservation involves controlling vapor pressure to prevent spoilage.
Measuring Vapor Pressure
Accurate measurement of vapor pressure is essential for scientific research and industrial processes. Various methods are used to determine vapor pressure.
- Manometers measure vapor pressure by comparing it to a known pressure.
- Barometers can indirectly measure vapor pressure by observing changes in atmospheric pressure.
- Dynamic method involves measuring the rate of evaporation and condensation in a controlled environment.
- Static method measures the equilibrium pressure in a closed system at a constant temperature.
- Effusion method calculates vapor pressure by observing the rate at which gas escapes through a small hole.
Interesting Facts About Vapor Pressure
Vapor pressure has some fascinating aspects that highlight its importance in everyday life and scientific phenomena.
- Sublimation occurs when a solid changes directly to a gas, bypassing the liquid phase, due to vapor pressure.
- Frost-free freezers use vapor pressure principles to prevent ice buildup.
- Pressure cookers increase vapor pressure to cook food faster at higher temperatures.
- Aerosol cans rely on vapor pressure to dispense contents evenly.
- Cloud formation involves vapor pressure as water vapor condenses into droplets.
The Final Word on Vapor Pressure
Understanding vapor pressure helps explain why liquids evaporate, how weather patterns form, and even why your soda goes flat. It’s all about the balance between molecules escaping a liquid and those returning. Higher temperatures mean more molecules escape, leading to higher vapor pressure. This concept is crucial in fields like meteorology, cooking, and even aviation. Knowing these facts can make you appreciate the science behind everyday phenomena. So, next time you see a puddle drying up or feel the humidity in the air, you’ll know vapor pressure is at work. Keep these facts in mind, and you’ll see the world through a more scientific lens. Understanding this concept not only satisfies curiosity but also enhances your grasp of the natural world.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
