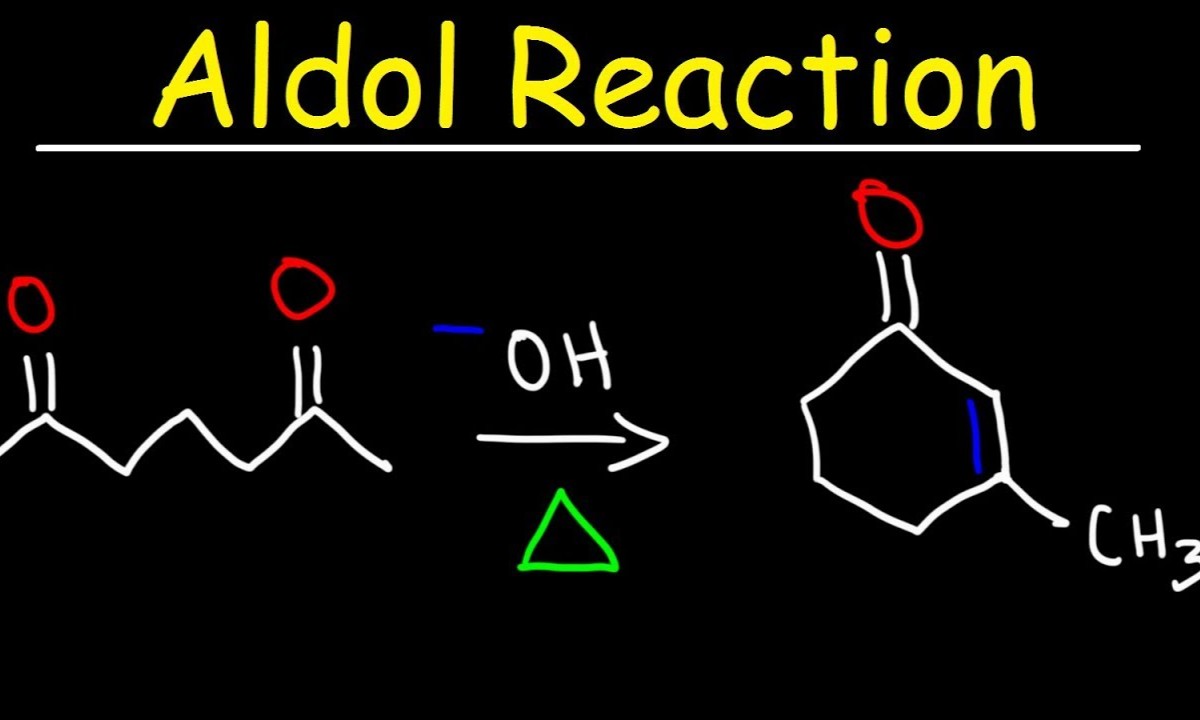
Aldol condensation is a fundamental reaction in organic chemistry, but what makes it so special? Aldol condensation involves the combination of two carbonyl compounds to form a larger β-hydroxy carbonyl compound, which then often dehydrates to yield an α,β-unsaturated carbonyl compound. This reaction is crucial for forming carbon-carbon bonds, making it a cornerstone in synthetic chemistry. Whether you're a student, a researcher, or just curious, understanding aldol condensation can open doors to grasping more complex chemical processes. Ready to dive into the world of aldol condensation? Here are 28 fascinating facts that will deepen your appreciation for this essential chemical reaction.
What is Aldol Condensation?
Aldol condensation is a fundamental reaction in organic chemistry. It involves the formation of a carbon-carbon bond between two carbonyl compounds, typically aldehydes or ketones. This reaction is crucial for creating complex molecules in both laboratory and industrial settings.
- Aldol condensation combines two molecules to form a larger one, often producing a β-hydroxy carbonyl compound.
- The reaction typically requires a base catalyst like hydroxide ions or an acid catalyst to proceed.
- Aldol stands for aldehyde and alcohol, reflecting the types of molecules involved in the reaction.
- The reaction can produce E/Z isomers, which are different spatial arrangements of the product molecules.
- Crossed aldol condensation occurs when two different carbonyl compounds react, expanding the diversity of possible products.
Mechanism of Aldol Condensation
Understanding the mechanism helps in controlling the reaction conditions and predicting the products. The mechanism involves several steps, each crucial for the reaction's success.
- The first step is the formation of an enolate ion from one of the carbonyl compounds.
- This enolate ion then attacks the carbonyl carbon of another molecule, forming a new carbon-carbon bond.
- The intermediate formed is a β-hydroxy carbonyl compound, which can further undergo dehydration.
- Dehydration removes a water molecule, leading to the formation of an α,β-unsaturated carbonyl compound.
- The reaction can be reversible, depending on the reaction conditions and the stability of the products.
Applications of Aldol Condensation
Aldol condensation is not just a laboratory curiosity; it has practical applications in various fields, including pharmaceuticals and materials science.
- Pharmaceuticals use aldol condensation to synthesize complex molecules like steroids and antibiotics.
- The reaction is crucial in the production of fragrances and flavoring agents.
- Polymer chemistry employs aldol condensation to create new materials with unique properties.
- In agrochemicals, the reaction helps in synthesizing pesticides and herbicides.
- Biochemistry utilizes aldol condensation in metabolic pathways, such as glycolysis.
Factors Affecting Aldol Condensation
Several factors can influence the outcome of aldol condensation, including temperature, solvent, and the nature of the reactants.
- Temperature can affect the rate and selectivity of the reaction.
- The choice of solvent can influence the solubility of reactants and the stability of intermediates.
- Steric hindrance from bulky groups can slow down or prevent the reaction.
- The electronic properties of the carbonyl compounds can affect their reactivity.
- Catalyst concentration can determine the rate and yield of the reaction.
Challenges and Limitations
Despite its usefulness, aldol condensation has some challenges and limitations that chemists must navigate.
- Side reactions can occur, leading to unwanted by-products.
- Enolate formation can be tricky, requiring precise control of reaction conditions.
- Dehydration can sometimes be incomplete, leaving β-hydroxy carbonyl compounds.
- Selectivity can be an issue, especially in crossed aldol condensations.
- Purification of the final product can be challenging due to the presence of multiple isomers.
Recent Advances in Aldol Condensation
Recent research has focused on improving the efficiency and selectivity of aldol condensation through various innovative approaches.
- Green chemistry approaches aim to make the reaction more environmentally friendly by using water as a solvent.
- Enzyme catalysis offers a way to achieve high selectivity and mild reaction conditions.
- Microwave-assisted aldol condensation can significantly speed up the reaction, reducing the time required for completion.
The Final Word on Aldol Condensation
Aldol condensation is a fascinating chemical reaction with significant applications in organic chemistry. It involves the formation of carbon-carbon bonds, making it essential for synthesizing complex molecules. This reaction is widely used in the pharmaceutical industry to create various drugs and in the production of fragrances and flavors. Understanding the basics of aldol condensation can provide valuable insights into the world of chemical synthesis.
Whether you're a student, a professional chemist, or just someone curious about chemistry, grasping the fundamentals of aldol condensation can open doors to new knowledge and opportunities. Keep exploring, experimenting, and learning about this remarkable reaction. Chemistry is full of wonders, and aldol condensation is just one of its many intriguing aspects. Happy experimenting!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
