Beryllium-8 is a fascinating isotope with unique properties that make it a subject of interest in both nuclear physics and astrophysics. Did you know that Beryllium-8 is highly unstable and has a half-life of only 8.19 x 10^-17 seconds? This means it decays almost instantly after being formed. Despite its fleeting existence, Beryllium-8 plays a crucial role in stellar nucleosynthesis, the process by which elements are formed in stars. Without Beryllium-8, the creation of heavier elements like carbon would be nearly impossible. This isotope also has intriguing implications for the study of fundamental forces in the universe. From its role in the triple-alpha process to its impact on our understanding of nuclear reactions, Beryllium-8 is a key player in the cosmic dance of elements.
What is Beryllium-8?
Beryllium-8 is a fascinating isotope of the element beryllium. It has unique properties and plays a significant role in nuclear physics and astrophysics. Let's dive into some intriguing facts about this isotope.
-
Beryllium-8 is highly unstable. It has an extremely short half-life of about 8.19 x 10^-17 seconds, making it one of the most unstable isotopes known.
-
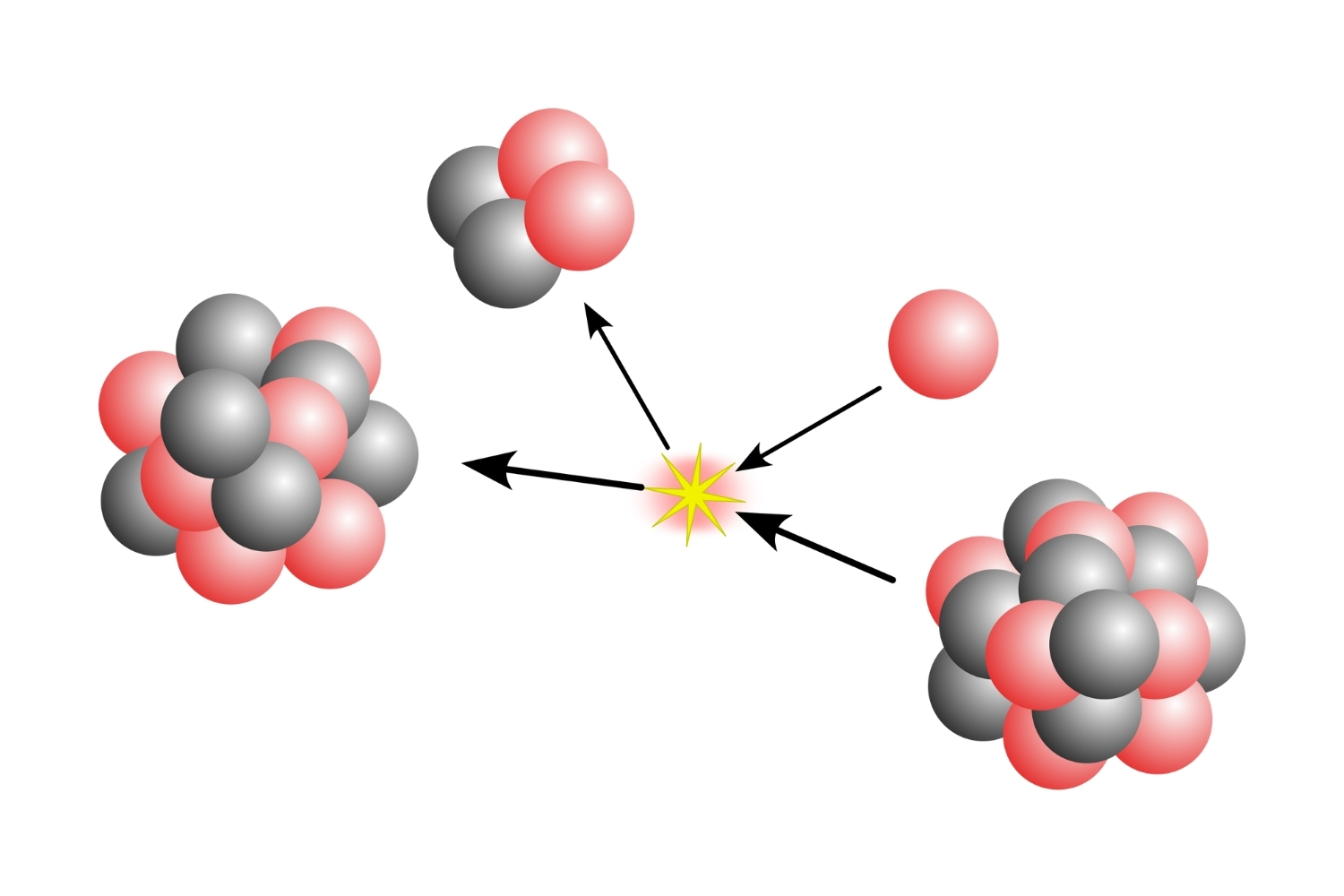
It decays into two alpha particles. Due to its instability, Beryllium-8 quickly decays into two helium-4 nuclei, also known as alpha particles.
-
Beryllium-8 is crucial in stellar nucleosynthesis. In stars, it forms temporarily during the triple-alpha process, which is essential for the creation of carbon-12.
-
It has an atomic number of 4. Like all beryllium isotopes, Beryllium-8 has four protons in its nucleus.
-
Beryllium-8 has four neutrons. This isotope contains an equal number of protons and neutrons, totaling eight nucleons.
The Role of Beryllium-8 in the Universe
Beryllium-8 might be fleeting, but its role in the cosmos is monumental. Here's how this isotope impacts the universe.
-
It acts as a bridge in the creation of heavier elements. During the triple-alpha process in stars, Beryllium-8 forms briefly, allowing the fusion of another helium-4 nucleus to create carbon-12.
-
Beryllium-8's instability limits its abundance. Its rapid decay means it doesn't accumulate in significant amounts in the universe.
-
It influences the balance of elements in stars. The fleeting existence of Beryllium-8 affects the rates at which heavier elements are formed in stellar cores.
-
Beryllium-8's decay releases energy. The decay process contributes to the energy output of stars, impacting their luminosity and lifecycle.
-
It plays a role in supernovae. During supernova explosions, the conditions can briefly stabilize Beryllium-8, influencing the synthesis of new elements.
Scientific Discoveries Involving Beryllium-8
Scientists have made several groundbreaking discoveries involving Beryllium-8. These findings have expanded our understanding of nuclear physics and the universe.
-
Beryllium-8 was first observed in the 1930s. Early nuclear experiments revealed its existence and instability.
-
It helped confirm the triple-alpha process. Observations of Beryllium-8 in stellar environments supported theories about the formation of carbon-12.
-
Beryllium-8's properties are studied using particle accelerators. High-energy collisions in accelerators recreate the conditions needed to observe this isotope.
-
It has been used to test nuclear models. The behavior of Beryllium-8 provides insights into the forces and interactions within atomic nuclei.
-
Beryllium-8's decay patterns help refine theoretical predictions. Comparing observed decay rates with theoretical models improves our understanding of nuclear physics.
Interesting Properties of Beryllium-8
Beyond its role in the universe and scientific discoveries, Beryllium-8 has some unique properties worth noting.
-
Beryllium-8 has a unique nuclear structure. Its nucleus is highly symmetric, with equal numbers of protons and neutrons.
-
It exhibits quantum tunneling. The rapid decay of Beryllium-8 involves quantum tunneling, where particles pass through energy barriers.
-
Beryllium-8's formation involves high temperatures. In stars, temperatures exceeding 100 million Kelvin are required to form this isotope.
-
It has a high binding energy per nucleon. Despite its instability, Beryllium-8's nucleons are tightly bound together.
-
Beryllium-8 is not found naturally on Earth. Its short half-life means it cannot be detected in natural terrestrial environments.
Beryllium-8 in Research and Technology
Beryllium-8's unique properties make it a subject of ongoing research and technological applications.
-
It is used in nuclear fusion research. Understanding Beryllium-8 helps scientists develop more efficient fusion reactions.
-
Beryllium-8's decay is studied for medical applications. Research into its decay products informs radiation therapy techniques.
-
It aids in the development of particle detectors. Beryllium-8's properties help improve the sensitivity and accuracy of particle detection equipment.
-
Beryllium-8 is used in astrophysical simulations. Simulating its behavior helps model stellar processes and the evolution of stars.
-
It contributes to the study of fundamental forces. Research on Beryllium-8 informs our understanding of the strong nuclear force and other fundamental interactions.
Fun Facts About Beryllium-8
Let's wrap up with some fun and quirky facts about this intriguing isotope.
-
Beryllium-8 is a fleeting visitor in the universe. Its existence is so brief that it's often described as a "ghost" isotope.
-
It has a role in science fiction. Some sci-fi stories feature Beryllium-8 in futuristic technologies and space travel scenarios.
-
Beryllium-8's decay is a cosmic fireworks show. The rapid decay into alpha particles is like a tiny, invisible explosion.
-
It has a unique place in the periodic table. Beryllium-8's properties make it a standout among the lighter elements.
-
Beryllium-8 is a challenge for scientists. Its instability and short lifespan make it difficult to study, adding to its mystique.
-
It has inspired art and literature. The enigmatic nature of Beryllium-8 has sparked the imagination of artists and writers.
-
Beryllium-8 is a key to understanding the universe. Despite its fleeting existence, it holds clues to the origins and evolution of the cosmos.
-
It is a testament to the power of nuclear forces. The formation and decay of Beryllium-8 showcase the incredible forces at work within atomic nuclei.
-
Beryllium-8 is a bridge between science and wonder. Its study connects the rigorous world of physics with the awe-inspiring mysteries of the universe.
-
It has a role in educational demonstrations. Beryllium-8's properties are used to teach students about nuclear physics and stellar processes.
-
Beryllium-8 is a reminder of the universe's complexity. This tiny isotope exemplifies the intricate and interconnected nature of the cosmos.
The Fascinating World of Beryllium-8
Beryllium-8 is a short-lived isotope with a half-life of just 8.19 x 10^-17 seconds. This fleeting existence makes it a key player in the universe's creation story. Its rapid decay into two alpha particles is crucial in the triple-alpha process, allowing stars to produce carbon, which is essential for life.
Despite its brief lifespan, Beryllium-8's role in nuclear fusion and stellar nucleosynthesis can't be overstated. It acts as a bridge in the formation of heavier elements, influencing the chemical makeup of the cosmos. This isotope's unique properties continue to intrigue scientists, offering insights into the fundamental processes that shape our universe.
Understanding Beryllium-8 helps us appreciate the delicate balance of forces and reactions that govern the stars. Its existence, though fleeting, leaves a lasting impact on the cosmic landscape, reminding us of the intricate dance of particles that sustain life.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
