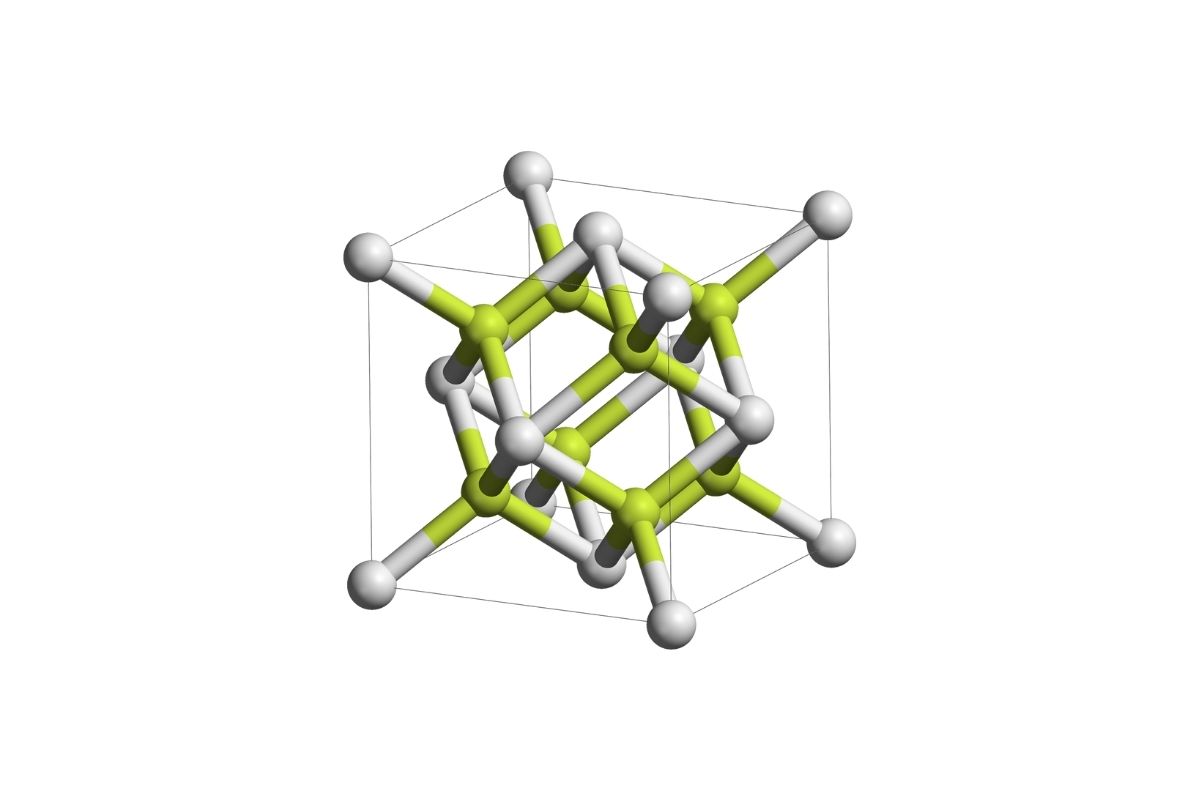
Sodium Helide might sound like a term from a sci-fi novel, but it's a real chemical compound with some intriguing properties. What exactly is Sodium Helide? Sodium Helide is a compound formed by the combination of sodium and helium, two elements you might recognize from table salt and party balloons. This compound is quite rare and forms under extreme conditions, such as high pressures and low temperatures. Scientists study it to understand more about chemical bonding and the behavior of elements under unusual conditions. Ready to learn more? Here are 25 fascinating facts about this unique compound that will blow your mind!
What is Sodium Helide?
Sodium Helide is a chemical compound that might sound unfamiliar to many. It's a fascinating subject with unique properties and applications. Let's dive into some intriguing facts about Sodium Helide.
-
Sodium Helide is a compound formed by the combination of sodium (Na) and helium (He).
-
This compound is extremely rare and not naturally occurring on Earth.
-
Sodium Helide is typically created in laboratory settings under very specific conditions.
-
The compound is known for its unusual bonding, which is not typical for most elements.
Properties of Sodium Helide
Understanding the properties of Sodium Helide can give us insight into why it's so unique. Here are some key properties that make this compound stand out.
-
Sodium Helide has a crystalline structure, which is quite rare for compounds involving noble gases like helium.
-
The bonding in Sodium Helide is ionic, which is unusual because helium is generally inert.
-
This compound is stable only under high pressure, making it difficult to study.
-
Sodium Helide is colorless and odorless, similar to its constituent elements.
Applications of Sodium Helide
While Sodium Helide is not commonly used, it has some interesting potential applications. Let's explore where this compound might be useful.
-
Sodium Helide could be used in high-pressure physics experiments to understand bonding under extreme conditions.
-
It might help in the study of noble gas chemistry, which is a relatively unexplored field.
-
This compound could potentially be used in advanced materials science for creating new materials with unique properties.
-
Sodium Helide might also have applications in quantum computing due to its unusual electronic properties.
How is Sodium Helide Created?
Creating Sodium Helide is no easy task. It requires precise conditions and advanced technology. Here's how scientists manage to create this rare compound.
-
Sodium Helide is typically synthesized using high-pressure techniques in a laboratory.
-
The process involves compressing sodium and helium together at pressures exceeding 1 million atmospheres.
-
Specialized equipment like diamond anvil cells are used to achieve the necessary conditions for synthesis.
-
The creation of Sodium Helide often requires temperatures close to absolute zero to maintain stability.
Challenges in Studying Sodium Helide
Studying Sodium Helide presents several challenges due to its unique properties and the conditions required for its stability. Here are some of the main obstacles scientists face.
-
The high-pressure conditions needed to stabilize Sodium Helide make it difficult to study using conventional methods.
-
Specialized equipment is required, which can be expensive and hard to maintain.
-
The compound's instability at normal pressures means it cannot be stored for long periods.
-
Researchers often have to work quickly and efficiently to gather data before the compound decomposes.
Interesting Facts about Sodium Helide
Beyond its scientific properties and applications, Sodium Helide has some fascinating trivia associated with it. Here are a few interesting tidbits.
-
Sodium Helide was first predicted theoretically before it was ever synthesized in a lab.
-
The compound challenges traditional notions of chemical bonding, especially involving noble gases.
-
Sodium Helide has sparked interest in the scientific community for its potential to reveal new aspects of chemistry.
-
Despite its rarity, Sodium Helide has been the subject of numerous research papers and studies.
-
The study of Sodium Helide continues to push the boundaries of what we know about chemical compounds and their behavior under extreme conditions.
Sodium Helide: A Quick Recap
Sodium Helide, a fascinating compound, has some pretty cool facts. It’s not just a mix of sodium and helium; it’s a rare gem in chemistry. This compound is formed under extreme conditions, making it a topic of interest for scientists. Its unique properties, like its potential use in superconductors, make it stand out. Sodium Helide’s formation involves high pressure and temperature, showcasing the wonders of chemical reactions. While it’s not something you’ll find in your kitchen, its study helps us understand more about elements and their interactions. So, next time you hear about Sodium Helide, remember it’s more than just a name; it’s a window into the incredible world of chemistry. Keep exploring and stay curious!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
