Rutherfordium is one of those elements that sparks curiosity. Named after the famous physicist Ernest Rutherford, this synthetic element holds a special place in the periodic table. But what makes it so intriguing? Rutherfordium is a transition metal with the atomic number 104, and it’s not something you’ll find lying around. Created in a lab, it’s a testament to human ingenuity and scientific progress. Ever wondered why it’s so rare or what its uses are? This blog post dives into 50 fascinating facts about Rutherfordium, from its discovery to its unique properties. Get ready to learn something new about this elusive element!
Key Takeaways:
- Rutherfordium, a synthetic element named after Ernest Rutherford, is highly radioactive and has no stable isotopes. It's used in nuclear physics research but has no practical applications due to its short half-life.
- Rutherfordium is produced in particle accelerators by bombarding lighter elements with heavy ions. Its discovery led to a naming controversy and it continues to intrigue scientists due to its unique properties influenced by relativistic effects.
What is Rutherfordium?
Rutherfordium, a synthetic element, holds a unique place in the periodic table. Named after the famous physicist Ernest Rutherford, it has intriguing properties and a fascinating history. Let's dive into some interesting facts about this element.
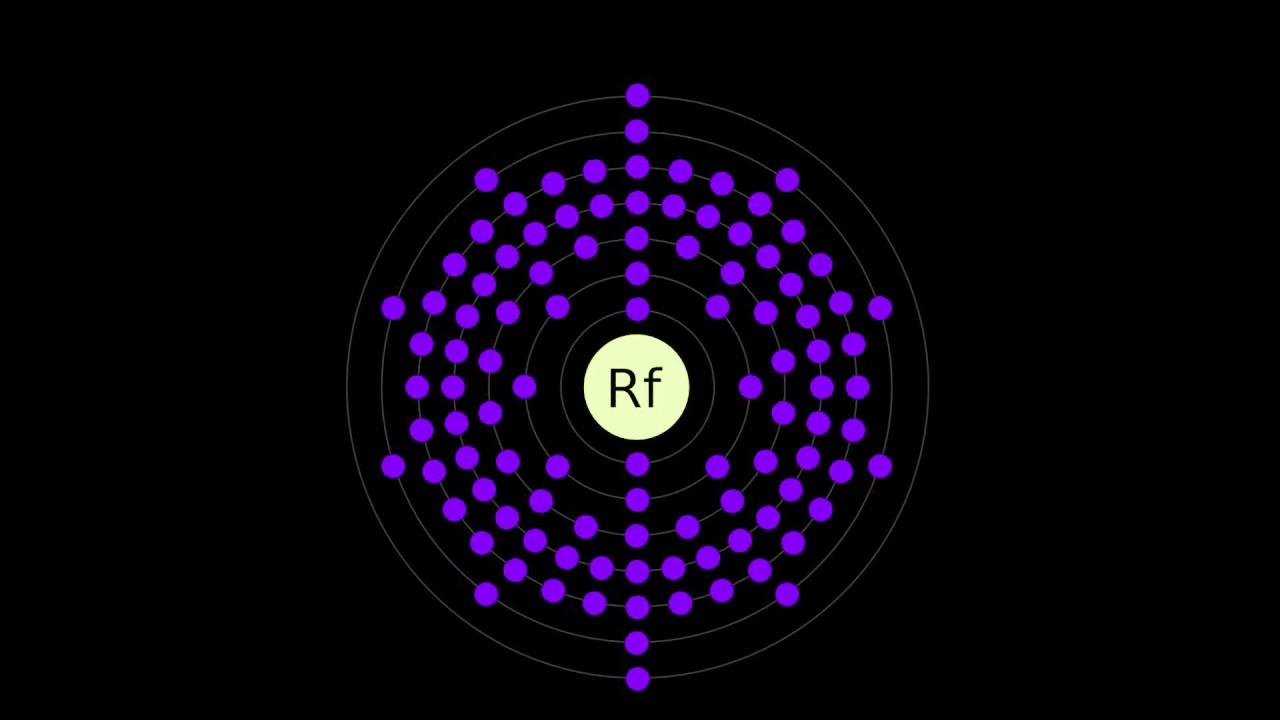
- Rutherfordium has the symbol Rf and atomic number 104.
- It belongs to the transition metals group.
- This element is synthetic, meaning it is not found naturally on Earth.
- Rutherfordium was first discovered in 1964 by a team of Soviet scientists.
- The discovery took place at the Joint Institute for Nuclear Research in Dubna, Russia.
- American scientists at the Lawrence Berkeley National Laboratory also claimed to have discovered it in 1969.
- The element was named after Ernest Rutherford, a pioneer in nuclear physics.
- Rutherfordium is highly radioactive.
- It has no stable isotopes.
- The most stable isotope, Rutherfordium-267, has a half-life of about 1.3 hours.
Properties of Rutherfordium
Understanding the properties of Rutherfordium helps us appreciate its unique characteristics. Here are some key properties:
- Rutherfordium is expected to be a solid at room temperature.
- It is predicted to have a metallic appearance.
- The element is part of the d-block in the periodic table.
- Rutherfordium's electron configuration is [Rn] 5f14 6d2 7s2.
- It is placed in period 7 of the periodic table.
- Rutherfordium is part of the group 4 elements.
- It shares similarities with hafnium and zirconium.
- The element has an estimated density of about 23 g/cm³.
- Rutherfordium's melting point is predicted to be around 2100°C.
- Its boiling point is estimated to be approximately 5500°C.
Uses and Applications
Despite being a synthetic element, Rutherfordium has potential uses in scientific research. Here are some facts about its applications:
- Rutherfordium is primarily used for research purposes.
- It helps scientists understand the properties of superheavy elements.
- The element is used in nuclear physics experiments.
- Rutherfordium's behavior provides insights into relativistic effects.
- It aids in studying the chemistry of heavy elements.
- The element's short half-life limits its practical applications.
- Rutherfordium is not used in commercial products.
- It has no known biological role.
- The element's radioactivity makes it hazardous to handle.
- Research on Rutherfordium contributes to the development of new elements.
Production and Synthesis
Creating Rutherfordium involves complex processes. Here are some interesting facts about its production:
- Rutherfordium is produced in particle accelerators.
- It is created by bombarding lighter elements with heavy ions.
- The element is typically synthesized using californium-249 and carbon-12.
- Rutherfordium can also be produced using plutonium-242 and neon-22.
- The production process requires high-energy collisions.
- Only a few atoms of Rutherfordium are produced at a time.
- The element's short half-life makes it challenging to study.
- Advanced detection techniques are needed to identify Rutherfordium.
- The synthesis of Rutherfordium helps refine nuclear theories.
- Research on its production contributes to the discovery of new elements.
Fun and Lesser-Known Facts
Rutherfordium has some fun and lesser-known aspects that make it even more intriguing. Here are a few:
- Rutherfordium was initially named kurchatovium by Soviet scientists.
- The name was later changed to honor Ernest Rutherford.
- The element's discovery led to a naming controversy between American and Soviet scientists.
- Rutherfordium is part of the transactinide series.
- It is one of the heaviest elements in the periodic table.
- The element's chemical properties are still being explored.
- Rutherfordium's compounds are not well-studied.
- It is predicted to form oxides and halides.
- The element's chemistry is influenced by relativistic effects.
- Rutherfordium continues to be a subject of scientific curiosity.
The Final Word on Rutherfordium
Rutherfordium, with its atomic number 104, stands as a testament to human curiosity and scientific progress. Discovered in the 1960s, this synthetic element has no stable isotopes and is created in labs through nuclear reactions. Named after Ernest Rutherford, it honors his groundbreaking work in nuclear physics. Though it has no practical applications yet, its study helps scientists understand the properties of heavy elements and the forces at play within atomic nuclei. Rutherfordium's fleeting existence, lasting only seconds, makes it a challenge to study, but each experiment brings us closer to unlocking the mysteries of the periodic table's heaviest members. As research continues, who knows what new insights this elusive element might reveal? For now, Rutherfordium remains a fascinating chapter in the story of chemistry, reminding us of the endless possibilities that lie within the atomic world.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


