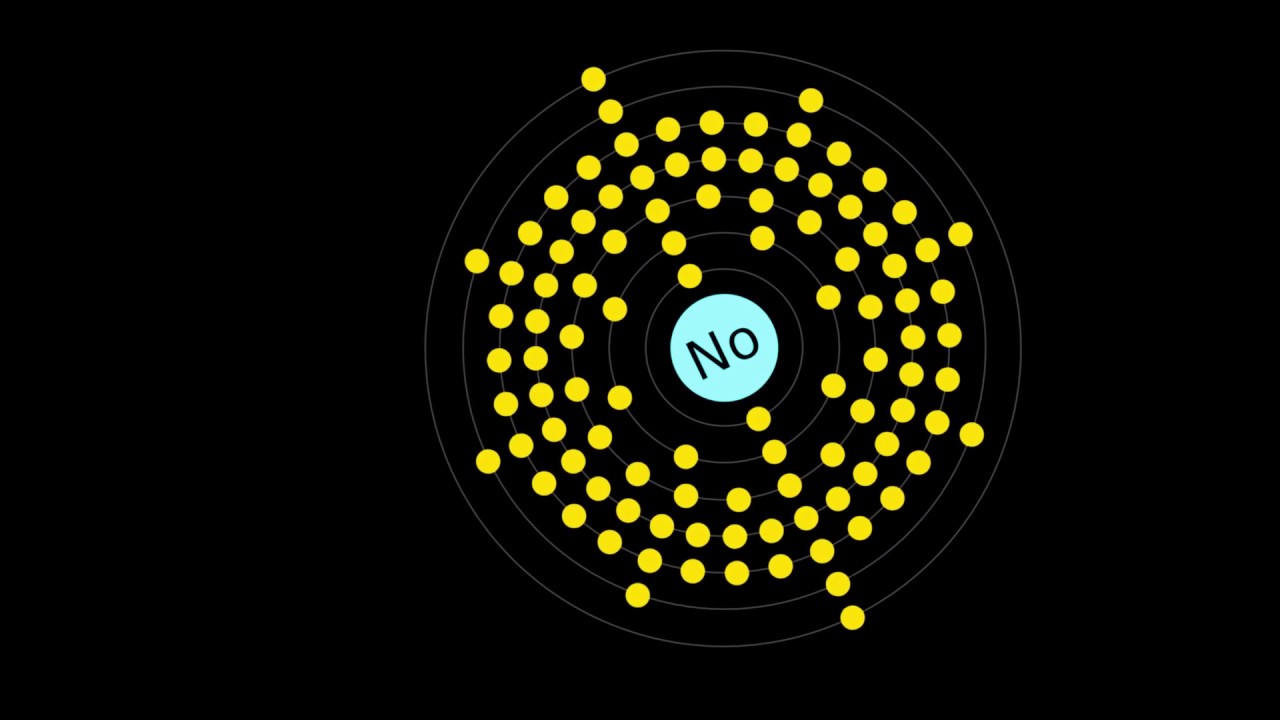
Nobelium, a synthetic element with the symbol No and atomic number 102, is part of the actinide series. Named after Alfred Nobel, the inventor of dynamite and founder of the Nobel Prize, this element is fascinating yet elusive. Did you know Nobelium is highly radioactive and has no stable isotopes? Discovered in the 1960s, it’s produced in minute amounts through nuclear reactions. Its most stable isotope, Nobelium-259, has a half-life of just 58 minutes. This means Nobelium doesn’t exist naturally on Earth. Scientists study it to understand more about the properties of heavy elements. Curious about Nobelium’s unique characteristics? Let’s dive into 50 intriguing facts about this rare element.
Key Takeaways:
- Nobelium is a rare, radioactive element named after Alfred Nobel. It has no practical uses outside of scientific research and is challenging to study due to its short half-life and high radioactivity.
- Despite its mysterious and elusive nature, Nobelium has made appearances in popular culture, including science fiction novels and video games. Its discovery was filled with controversies, making it a topic of interest for science enthusiasts.
What is Nobelium?
Nobelium is a synthetic element with the symbol No and atomic number 102. It is part of the actinide series and is named after Alfred Nobel, the inventor of dynamite and founder of the Nobel Prize. Here are some fascinating facts about this elusive element.
- Nobelium was first discovered in 1957 by a team of scientists at the Nobel Institute of Physics in Stockholm, Sweden.
- The element was named in honor of Alfred Nobel, the famous Swedish chemist and inventor.
- Nobelium is a synthetic element, meaning it is not found naturally on Earth and must be created in a laboratory.
- It was first produced by bombarding curium-244 with carbon-12 ions.
- Nobelium is highly radioactive and has no stable isotopes.
- The most stable isotope of Nobelium is Nobelium-259, which has a half-life of about 58 minutes.
- Due to its short half-life, Nobelium has no practical applications outside of scientific research.
- Nobelium is part of the actinide series, which includes elements with atomic numbers from 89 to 103.
- It is the tenth transuranium element, meaning it has an atomic number greater than uranium (92).
- Nobelium is a metal, but its physical properties are not well known due to its rarity and radioactivity.
Discovery and Naming
The journey to discovering Nobelium was filled with challenges and controversies. Let's dive into some key moments in its history.
- The discovery of Nobelium was initially claimed by a team of scientists at the Nobel Institute of Physics in 1957.
- However, their findings were later disputed by a team of American scientists at the Lawrence Berkeley National Laboratory.
- In 1966, the International Union of Pure and Applied Chemistry (IUPAC) officially credited the discovery to a team of Soviet scientists at the Joint Institute for Nuclear Research in Dubna, Russia.
- The element was named Nobelium in honor of Alfred Nobel, despite the controversies surrounding its discovery.
- The symbol "No" was chosen for Nobelium to avoid confusion with the element nitrogen, which has the symbol "N."
Properties and Characteristics
Nobelium's properties are not well understood due to its rarity and radioactivity. However, scientists have gathered some information about this mysterious element.
- Nobelium is a metal, but its appearance is unknown because only microscopic amounts have been produced.
- It is expected to have a silvery-white or metallic appearance, similar to other actinides.
- Nobelium is highly radioactive, making it difficult to study its physical and chemical properties.
- It is believed to have a density of around 9.9 grams per cubic centimeter.
- Nobelium is expected to be a solid at room temperature, like other actinide metals.
Isotopes and Stability
Nobelium has several isotopes, but none of them are stable. Here are some interesting facts about its isotopes and their stability.
- Nobelium has 12 known isotopes, ranging from Nobelium-250 to Nobelium-262.
- The most stable isotope of Nobelium is Nobelium-259, with a half-life of about 58 minutes.
- Nobelium-255 has a half-life of about 3.1 minutes, making it one of the longer-lived isotopes.
- The shortest-lived isotope of Nobelium is Nobelium-250, with a half-life of only 0.25 seconds.
- Due to their short half-lives, Nobelium isotopes decay quickly into lighter elements through alpha decay.
Production and Uses
Producing Nobelium is a complex and expensive process, and its uses are limited to scientific research. Here are some facts about its production and uses.
- Nobelium is produced by bombarding lighter elements with heavy ions in a particle accelerator.
- The most common method of producing Nobelium involves bombarding curium-244 with carbon-12 ions.
- Due to its short half-life and radioactivity, Nobelium has no practical applications outside of scientific research.
- Nobelium is used in research to study the properties of heavy elements and their interactions.
- The production of Nobelium is limited to a few specialized laboratories around the world.
Fun Facts
Nobelium may be a mysterious and elusive element, but it has some fun and interesting facts worth sharing.
- Nobelium is one of the rarest elements on Earth, with only a few atoms produced at a time.
- The element's name was chosen to honor Alfred Nobel, the inventor of dynamite and founder of the Nobel Prize.
- Nobelium is part of the actinide series, which includes elements like uranium, plutonium, and americium.
- The discovery of Nobelium was filled with controversies and disputes among scientists from different countries.
- Nobelium's symbol "No" is one of the shortest element symbols in the periodic table.
Nobelium in Popular Culture
While Nobelium may not be well-known to the general public, it has made a few appearances in popular culture.
- Nobelium is sometimes mentioned in science fiction novels and movies as a rare and powerful element.
- In the video game "Fallout 3," Nobelium is referenced as a component of a fictional weapon called the "Nobelium Bomb."
- Nobelium has been featured in educational programs and documentaries about the periodic table and the discovery of new elements.
- The element's name and symbol have been used in various puzzles and trivia games related to chemistry and the periodic table.
- Nobelium's mysterious and elusive nature has made it a topic of interest for science enthusiasts and researchers alike.
Nobelium's Place in the Periodic Table
Nobelium holds a unique position in the periodic table as part of the actinide series. Here are some facts about its place in the periodic table.
- Nobelium is located in period 7 of the periodic table, among the actinide series.
- It has an atomic number of 102, making it the tenth transuranium element.
- Nobelium is positioned between mendelevium (101) and lawrencium (103) in the periodic table.
- The element's electron configuration is [Rn] 5f14 7s2, indicating its position in the actinide series.
- Nobelium's place in the periodic table reflects its similarities to other actinide elements in terms of chemical properties.
Challenges in Studying Nobelium
Studying Nobelium presents numerous challenges due to its rarity and radioactivity. Here are some facts about the difficulties scientists face when researching this element.
- Nobelium's short half-life makes it difficult to study its physical and chemical properties in detail.
- The element's high radioactivity poses safety risks for researchers working with it.
- Producing Nobelium requires specialized equipment and facilities, limiting the number of laboratories that can study it.
- The small amounts of Nobelium produced make it challenging to conduct experiments and gather data.
- Despite these challenges, scientists continue to study Nobelium to learn more about the properties of heavy elements and their interactions.
Nobelium's Fascinating World
Nobelium, element 102 on the periodic table, holds a unique spot in science. Discovered in the 20th century, it’s named after Alfred Nobel, the inventor of dynamite and founder of the Nobel Prize. This synthetic element is part of the actinide series and is highly radioactive. Scientists create it in labs by bombarding curium with carbon ions. Nobelium has no significant commercial applications due to its short half-life and scarcity. However, its study helps researchers understand more about nuclear reactions and the properties of heavy elements. Despite its limited practical use, Nobelium’s discovery and ongoing research highlight human curiosity and the quest for knowledge. It’s a reminder of how even the most obscure elements can contribute to our understanding of the universe. So, next time you glance at the periodic table, remember the intriguing story behind Nobelium.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.