Plutonium is one of the most intriguing elements on the periodic table. Known for its role in nuclear power and weapons, this heavy metal has a complex story. Did you know plutonium was discovered during World War II? It’s not just a scientific curiosity; it’s a key player in both energy production and historical events. Plutonium can be found in trace amounts in nature, but it’s mostly produced in reactors. Its isotopes have unique properties that make them useful—and dangerous. From its silvery appearance to its radioactive nature, plutonium is a subject worth exploring. Ready to learn 50 fascinating facts about this powerful element? Let’s dive in!
Key Takeaways:
- Plutonium, discovered in 1940, is a dense, silvery-gray metal with unique properties. It's used in nuclear power, space exploration, and even glows in the dark!
- Plutonium is highly toxic and radioactive, posing environmental and health risks. Its role in history, science, and potential for future technologies makes it a fascinating but controversial element.
What is Plutonium?
Plutonium is a fascinating element with a rich history and unique properties. It's a heavy metal used in various applications, from nuclear power to scientific research. Let's dive into some intriguing facts about this element.
-
Plutonium was discovered in 1940 by scientists Glenn T. Seaborg, Edwin McMillan, Joseph W. Kennedy, and Arthur C. Wahl at the University of California, Berkeley.
-
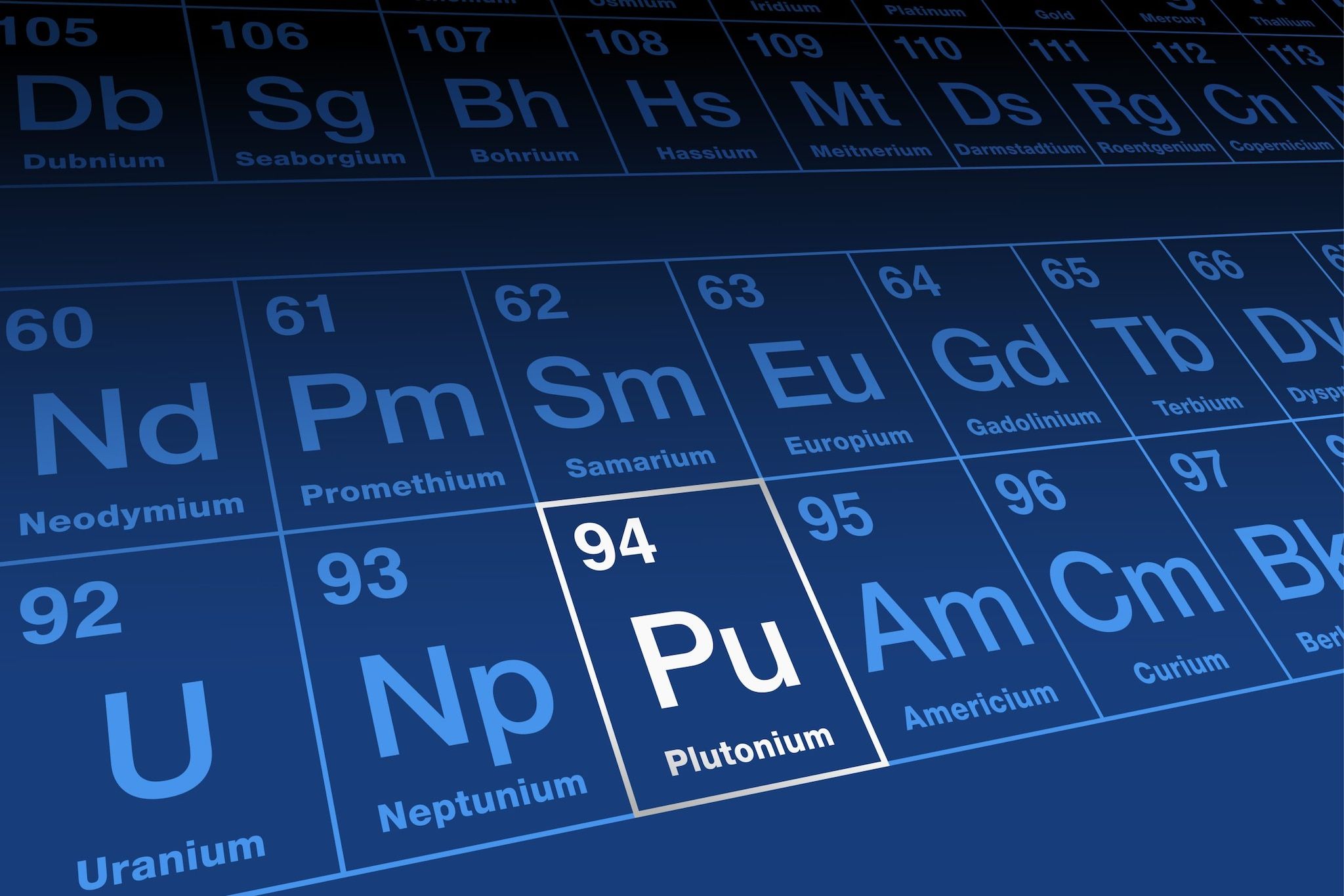
Named after the dwarf planet Pluto, plutonium follows uranium and neptunium in the periodic table, which were named after Uranus and Neptune.
-
Plutonium is a radioactive element with the symbol Pu and atomic number 94.
-
It is a silvery-gray metal that tarnishes when exposed to air, forming a dull coating.
-
Plutonium has six allotropes, or different structural forms, which change with temperature and pressure.
-
The most stable isotope of plutonium is Pu-244, with a half-life of about 80 million years.
-
Pu-239 is the most commonly used isotope in nuclear reactors and weapons due to its ability to sustain a chain reaction.
-
Plutonium is highly toxic and poses significant health risks if inhaled or ingested.
-
It is a key component in nuclear weapons, providing the explosive power in atomic bombs.
-
Plutonium can be used as a fuel in nuclear reactors, particularly in mixed oxide (MOX) fuel.
Plutonium in Science and Technology
Plutonium's unique properties make it valuable in various scientific and technological applications. Here are some more facts about its uses and characteristics.
-
Plutonium-238 is used in radioisotope thermoelectric generators (RTGs), which power spacecraft like the Voyager probes and the Curiosity rover.
-
RTGs convert heat from radioactive decay into electricity, providing long-lasting power for missions far from the Sun.
-
Plutonium's density is about 19.86 g/cm³, making it one of the densest elements on Earth.
-
It has a melting point of 640°C (1184°F) and a boiling point of 3228°C (5842°F).
-
Plutonium can form compounds with other elements, such as plutonium dioxide (PuO₂) and plutonium chloride (PuCl₃).
-
It exhibits multiple oxidation states, ranging from +3 to +7, which affect its chemical behavior.
-
Plutonium's half-life varies by isotope, with Pu-239 having a half-life of 24,100 years.
-
It is produced in nuclear reactors by bombarding uranium-238 with neutrons.
-
Plutonium can be recycled from spent nuclear fuel, reducing the need for new uranium mining.
-
Its use in nuclear power helps reduce greenhouse gas emissions by providing a low-carbon energy source.
Plutonium in History and Culture
Plutonium has played a significant role in history and has even made its way into popular culture. Here are some interesting historical and cultural facts.
-
The first atomic bomb, "Trinity," used plutonium and was detonated on July 16, 1945, in New Mexico.
-
The bomb dropped on Nagasaki, "Fat Man," also used plutonium and contributed to the end of World War II.
-
Plutonium has been a subject of controversy, particularly regarding nuclear weapons proliferation and environmental contamination.
-
The Cold War saw a significant increase in plutonium production, as both the US and the Soviet Union built up their nuclear arsenals.
-
Plutonium has appeared in movies and TV shows, such as "Back to the Future," where it powers the DeLorean time machine.
-
It has been referenced in literature, including Tom Clancy's novels and other works of fiction.
-
Plutonium's discovery earned Glenn T. Seaborg a share of the 1951 Nobel Prize in Chemistry.
-
The element has been the focus of numerous scientific studies, advancing our understanding of nuclear physics and chemistry.
-
Plutonium's role in space exploration has been crucial, powering missions to the outer planets and beyond.
-
Its potential for future energy solutions continues to be explored, with research into advanced nuclear reactors and fuel cycles.
Environmental and Health Concerns
While plutonium has many uses, it also poses significant environmental and health risks. Here are some facts about these concerns.
-
Plutonium is highly radioactive, emitting alpha particles that can damage living tissue.
-
Inhalation of plutonium particles can lead to lung cancer and other serious health issues.
-
Plutonium contamination in the environment can persist for thousands of years due to its long half-life.
-
Nuclear accidents, such as Chernobyl and Fukushima, have released plutonium into the environment, raising concerns about long-term impacts.
-
Plutonium waste requires secure storage, often in deep geological repositories, to prevent contamination.
-
The US and Russia have agreed to reduce their plutonium stockpiles, converting excess material into MOX fuel or other forms.
-
Plutonium's toxicity necessitates strict safety protocols in handling and transport to protect workers and the public.
-
Environmental cleanup of plutonium-contaminated sites is a complex and costly process, often involving advanced technologies.
-
Plutonium's presence in the environment can be detected using sophisticated instruments, such as mass spectrometers.
-
Research into safer handling and disposal methods continues, aiming to minimize the risks associated with plutonium.
Fun and Lesser-Known Facts
Beyond its serious applications and risks, plutonium has some fun and lesser-known aspects. Here are a few more facts to round out our list.
-
Plutonium glows in the dark, emitting a faint blue or green light due to its radioactivity.
-
It is one of the few elements that can undergo fission, splitting into smaller atoms and releasing energy.
-
Plutonium's discovery was kept secret during World War II as part of the Manhattan Project.
-
The element can be used to create superheavy elements, such as livermorium, through nuclear reactions.
-
Plutonium has been used in scientific experiments to study the properties of matter under extreme conditions.
-
It can form alloys with other metals, such as gallium, to stabilize its structure.
-
Plutonium's unique properties make it a subject of ongoing research, with scientists continually uncovering new insights.
-
The element has a complex crystal structure, which changes with temperature and pressure.
-
Plutonium's role in nuclear power has sparked debates about the future of energy and the environment.
-
Its potential for new technologies, such as advanced reactors and space exploration, keeps plutonium at the forefront of scientific innovation.
Plutonium's Impact and Future
Plutonium, a fascinating element, has shaped history and science in profound ways. From its discovery during the Manhattan Project to its role in nuclear power and weapons, this heavy metal has left a significant mark. Its radioactive properties make it both a powerful energy source and a hazardous material, requiring careful handling and disposal.
Despite its dangers, plutonium's potential for generating electricity in nuclear reactors offers a cleaner alternative to fossil fuels. However, the challenges of managing its long-lived radioactive waste remain a critical issue. Advances in technology and stricter regulations aim to mitigate these risks, ensuring safer use and storage.
Understanding plutonium's complexities helps us appreciate its contributions and the need for responsible stewardship. As we continue to explore its capabilities, balancing its benefits with safety concerns will be crucial for a sustainable future.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.